A terminal α3-galactose modification regulates an E3 ubiquitin ligase subunit in Toxoplasma gondii
- PMID: 32414843
- PMCID: PMC7335778
- DOI: 10.1074/jbc.RA120.013792
A terminal α3-galactose modification regulates an E3 ubiquitin ligase subunit in Toxoplasma gondii
Abstract
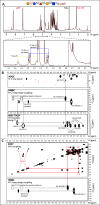
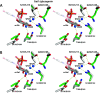
Skp1, a subunit of E3 Skp1/Cullin-1/F-box protein ubiquitin ligases, is modified by a prolyl hydroxylase that mediates O2 regulation of the social amoeba Dictyostelium and the parasite Toxoplasma gondii The full effect of hydroxylation requires modification of the hydroxyproline by a pentasaccharide that, in Dictyostelium, influences Skp1 structure to favor assembly of Skp1/F-box protein subcomplexes. In Toxoplasma, the presence of a contrasting penultimate sugar assembled by a different glycosyltransferase enables testing of the conformational control model. To define the final sugar and its linkage, here we identified the glycosyltransferase that completes the glycan and found that it is closely related to glycogenin, an enzyme that may prime glycogen synthesis in yeast and animals. However, the Toxoplasma enzyme catalyzes formation of a Galα1,3Glcα linkage rather than the Glcα1,4Glcα linkage formed by glycogenin. Kinetic and crystallographic experiments showed that the glycosyltransferase Gat1 is specific for Skp1 in Toxoplasma and also in another protist, the crop pathogen Pythium ultimum The fifth sugar is important for glycan function as indicated by the slow-growth phenotype of gat1Δ parasites. Computational analyses indicated that, despite the sequence difference, the Toxoplasma glycan still assumes an ordered conformation that controls Skp1 structure and revealed the importance of nonpolar packing interactions of the fifth sugar. The substitution of glycosyltransferases in Toxoplasma and Pythium by an unrelated bifunctional enzyme that assembles a distinct but structurally compatible glycan in Dictyostelium is a remarkable case of convergent evolution, which emphasizes the importance of the terminal α-galactose and establishes the phylogenetic breadth of Skp1 glycoregulation.
Keywords: E3 ubiquitin ligase; NMR; Pythium; SCF; Skp1; Toxoplasma; Toxoplasma gondii; X-ray crystallography; cytoplasmic glycosylation; glycogenin; glycosyltransferase; molecular dynamics; molecular dynamics simulation; nuclear magnetic resonance (NMR); post-translational modification; post-translational modification (PTM); s: X-ray crystallography.
© 2020 Mandalasi et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures








References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

