Brentuximab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone as frontline treatment for patients with CD30-positive B-cell lymphomas
- PMID: 32414850
- PMCID: PMC8168499
- DOI: 10.3324/haematol.2019.238675
Brentuximab vedotin in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone as frontline treatment for patients with CD30-positive B-cell lymphomas
Abstract
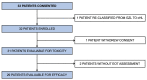
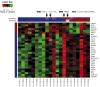
We conducted a phase I/II multicenter trial using 6 cycles of brentuximab vedotin (BV) in combination with rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHP) for treatment of patients with CD30-positive (+) B-cell lymphomas. Thirty-one patients were evaluable for toxicity and 29 for efficacy including 22 with primary mediastinal B-cell lymphoma (PMBCL), 5 with diffuse large B-cell lymphoma (DLBCL), and 2 with gray zone lymphoma (GZL). There were no treatment-related deaths; 32% of patients had non-hematological grade 3/4 toxicities. The overall response rate was 100% (95% CI: 88-100) with 86% (95% CI: 68-96) of patients achieving complete response at the end of systemic treatment. Consolidative radiation following end of treatment response assessment was permissible and used in 52% of all patients including 59% of patients with PMBCL. With a median follow-up of 30 months, the 2-year progression-free survival (PFS) and overall survival (OS) were 85% (95% CI: 66-94) and 100%, respectively. In the PMBCL cohort, 2-year PFS was 86% (95% CI: 62-95). In summary, BV-R-CHP with or without consolidative radiation is a feasible and active frontline regimen for CD30+ B-cell lymphomas (NCT01994850).
Figures


References
-
- Younes A, Yasothan U, Kirkpatrick P. Brentuximab vedotin. Nat Rev Drug Discov. 2012;11:19. - PubMed
-
- Pro B, Advani R, Brice P, et al. . Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190-2196. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

