Venetoclax combines synergistically with FLT3 inhibition to effectively target leukemic cells in FLT3-ITD+ acute myeloid leukemia models
- PMID: 32414851
- PMCID: PMC8017817
- DOI: 10.3324/haematol.2019.244020
Venetoclax combines synergistically with FLT3 inhibition to effectively target leukemic cells in FLT3-ITD+ acute myeloid leukemia models
Abstract
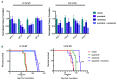
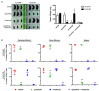
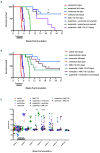
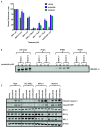
FLT3 internal tandem duplication (FLT3-ITD) mutations account for ~25% of adult acute myeloid leukemia cases and are associated with poor prognosis. Venetoclax, a selective BCL-2 inhibitor, has limited monotherapy activity in relapsed/refractory acute myeloid leukemia with no responses observed in a small subset of FLT3-ITD+ patients. Further, FLT3-ITD mutations emerged at relapse following venetoclax monotherapy and combination therapy suggesting a potential mechanism of resistance. Therefore, we investigated the convergence of FLT3-ITD signaling on the BCL-2 family proteins and determined combination activity of venetoclax and FLT3-ITD inhibition in preclinical models. In vivo, venetoclax combined with quizartinib, a potent FLT3 inhibitor, showed greater anti-tumor efficacy and prolonged survival compared to monotherapies. In a patient-derived FLT3-ITD+ xenograft model, cotreatment with venetoclax and quizartinib at clinically relevant doses had greater anti-tumor activity in the tumor microenvironment compared to quizartinib or venetoclax alone. Use of selective BCL-2 family inhibitors further identified a role for BCL-2, BCL-XL and MCL-1 in mediating survival in FLT3-ITD+ cells in vivo and highlighted the need to target all three proteins for greatest anti-tumor activity. Assessment of these combinations in vitro revealed synergistic combination activity for quizartinib and venetoclax but not for quizartinib combined with BCL-XL or MCL-1 inhibition. FLT3-ITD inhibition was shown to indirectly target both BCL-XL and MCL-1 through modulation of protein expression, thereby priming cells toward BCL-2 dependence for survival. These data demonstrate that FLT3-ITD inhibition combined with venetoclax has impressive anti-tumor activity in FLT3-ITD+ acute myeloid leukemia preclinical models and provides strong mechanistic rational for clinical studies.
Figures



References
-
- Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17(9):1738-1752. - PubMed
-
- Yamamoto Y, Kiyoi H, Nakano Y, et al. . Activation mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97(8):2434-2439. - PubMed
-
- Choudhary C, Muller-Tidow C, Berdel WE, Serve H. Signal transduction of oncogenic Flt3. Int J Hematol. 2005;82(2):93-99. - PubMed
-
- Hayakawa F, Towatari M, Kiyoi H, et al. . Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL3- dependent cell lines. Oncogene. 2000; 19:624-631. - PubMed
-
- Mizuki M, Fenski R, Halfter H, et al. . Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood. 2000;96(12):3907-3914. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

