The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation
- PMID: 32414858
- PMCID: PMC7239569
- DOI: 10.1136/jitc-2019-000155
The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation
Erratum in
-
Correction: The society for immunotherapy of cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation.J Immunother Cancer. 2020 Jun;8(1):e000155corr1. doi: 10.1136/jitc-2019-000155corr1. J Immunother Cancer. 2020. PMID: 32499296 Free PMC article. No abstract available.
Abstract
Objectives: The interaction between the immune system and tumor cells is an important feature for the prognosis and treatment of cancer. Multiplex immunohistochemistry (mIHC) and multiplex immunofluorescence (mIF) analyses are emerging technologies that can be used to help quantify immune cell subsets, their functional state, and their spatial arrangement within the tumor microenvironment.
Methods: The Society for Immunotherapy of Cancer (SITC) convened a task force of pathologists and laboratory leaders from academic centers as well as experts from pharmaceutical and diagnostic companies to develop best practice guidelines for the optimization and validation of mIHC/mIF assays across platforms.
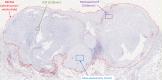
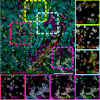
Results: Representative outputs and the advantages and disadvantages of mIHC/mIF approaches, such as multiplexed chromogenic IHC, multiplexed immunohistochemical consecutive staining on single slide, mIF (including multispectral approaches), tissue-based mass spectrometry, and digital spatial profiling are discussed.
Conclusions: mIHC/mIF technologies are becoming standard tools for biomarker studies and are likely to enter routine clinical practice in the near future. Careful assay optimization and validation will help ensure outputs are robust and comparable across laboratories as well as potentially across mIHC/mIF platforms. Quantitative image analysis of mIHC/mIF output and data management considerations will be addressed in a complementary manuscript from this task force.
Keywords: image analysis; immunology; oncology; tumors.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JMT has served on an advisory board, consulting and received research funding from Bristol-Myers Squibb; is a consultant and an advisory board member for Merck and Co., Inc., Kenilworth, New Jersey, USA, Amgen and AstraZeneca. MA is a founder, consultant and board member of IONpath. SG (Gnjatic) is a co-inventor on an issued patent that is filed through Icahn School of Medicine at Mount Sinai (ISMMS) and non-exclusively licensed to Caprion; reports consultancy/advisory fees from Merck and Co., Inc., Kenilworth, New Jersey, USA, Neon Therapeutics and OncoMed Pharmaceutical; and has received research funding from Agenus, Bristol-Myers Squibb, Genentech, Immune Design, Janssen R&D, Pfizer, Regeneron and Takeda Pharmaceutical. CVH is a stockholder of Bristol-Myers Squibb. TJH has received research support from Bristol-Myers Squibb and General Electric. JJ is an employee of Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA and stockowner of Merck & Co., Inc., Kenilworth, New Jersey, USA; and a stockholder of Regeneron Pharmaceuticals, Illumina and Google. MCR is an employee and stockholder of AstraZeneca. DLR is a consultant, advisor, or serves on a scientific advisory board for Amgen, AstraZeneca, Biocept, Bristol-Myers Squibb, Cell Signaling Technology, Cepheid, Daiichi Sankyo, GlaxoSmithKline, Invicro Konica Minolta, Merck and Co., Inc., Kenilworth, New Jersey, USA, NanoString, PerkinElmer, Ultivue and Ventana; is a founder and equity holder of PixelGear; has received research support or instrument support from AstraZeneca, Cepheid, Eli Lilly, Navigate BioPharma/Novartis, NextCure, Ultivue, Akoya Biosciences/PerkinElmer, NanoString and Ventana; and has received royalty from Rarecyte. JRC is an employee and unvested stock options holder of AstraZeneca. KAS has received consultant honoraria from Clinica Alemana de Santiago, Celgene, Moderna Therapeutics, Pierre-Fabre Research Institute, Shattuck Labs, AbbVie, AstraZeneca, EMD Serono, Ono Pharmaceuticals, Dynamo Therapeutics and Takeda Pharmaceutical; speaker honoraria from Merck & Co., Inc., Kenilworth, New Jersey, USA, Takeda Pharmaceutical, The PeerView Institute and Fluidigm; and research funding from Genoptix/Navigate BioPharma (Novartis), Tioma Therapeutics (formerly Vasculox), Tesaro, Moderna, Takeda Pharmaceutical, Surface Oncology, Pierre-Fabre Research Institute, Merck & Co., Inc., Kenilworth, New Jersey, USA, Bristol-Myers Squibb, AstraZeneca and Eli Lilly. ECS is an employee of Jounce Therapeutics. CSF is an employee of Roche Diagnostics GmbH. KK is an employee and shareholder of Roche. SJR receives research grant support from Bristol-Myers Squibb, Merck and Co., Inc., Kenilworth, New Jersey, USA, Kite/Gilead and Affimed Inc. ES is an employee of AstraZeneca. KES is an employee and stockholder of AstraZeneca; and a spouse of an employee of Arcellx. MJS is an employee and stockholder of AstraZeneca. MTT has served on an advisory board for Myriad Genetics, Seattle Genetics and Novartis; and has presented as a speaker for NanoString. IIW has served on an advisory board for as well as received research support from Genetech/Roche, Bayer AG, Bristol-Myers Squibb, AstraZeneca, Pfizer, HTG Molecular Diagnostics and Merck and Co., Inc., Kenilworth, New Jersey, USA; has served on an advisory board for Asuragen, GlaxoSmithKline, Guardant Health and Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA; has received research support from DEPArray/Menarini Silicon Biosystems, Adaptive Biotechnologies, Adaptimmune, EMD Serono, Takeda Pharmaceutical, Amgen, Karus Therapeutics, Johnson & Johnson, Iovance Biotherapeutics, 4D pharma, Novartis, Akoya Biosciences; and has presented as a speaker for Genetech/Roche, Pfizer and Merck Sharp & Dohme, a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, USA. CBB has served on the scientific advisory boards at Bristol-Myers Squibb, Roche, PrimeVax and Halio DX; is a stockholder of PrimeVax and holds the patent US20180322632A1.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
