Tibetan PHD2, an allele with loss-of-function properties
- PMID: 32414920
- PMCID: PMC7275716
- DOI: 10.1073/pnas.1920546117
Tibetan PHD2, an allele with loss-of-function properties
Abstract
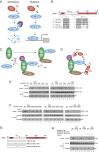
Tibetans have adapted to the chronic hypoxia of high altitude and display a distinctive suite of physiologic adaptations, including augmented hypoxic ventilatory response and resistance to pulmonary hypertension. Genome-wide studies have consistently identified compelling genetic signatures of natural selection in two genes of the Hypoxia Inducible Factor pathway, PHD2 and HIF2A The product of the former induces the degradation of the product of the latter. Key issues regarding Tibetan PHD2 are whether it is a gain-of-function or loss-of-function allele, and how it might contribute to high-altitude adaptation. Tibetan PHD2 possesses two amino acid changes, D4E and C127S. We previously showed that in vitro, Tibetan PHD2 is defective in its interaction with p23, a cochaperone of the HSP90 pathway, and we proposed that Tibetan PHD2 is a loss-of-function allele. Here, we report that additional PHD2 mutations at or near Asp-4 or Cys-127 impair interaction with p23 in vitro. We find that mice with the Tibetan Phd2 allele display augmented hypoxic ventilatory response, supporting this loss-of-function proposal. This is phenocopied by mice with a mutation in p23 that abrogates the PHD2:p23 interaction. Hif2a haploinsufficiency, but not the Tibetan Phd2 allele, ameliorates hypoxia-induced increases in right ventricular systolic pressure. The Tibetan Phd2 allele is not associated with hemoglobin levels in mice. We propose that Tibetans possess genetic alterations that both activate and inhibit selective outputs of the HIF pathway to facilitate successful adaptation to the chronic hypoxia of high altitude.
Keywords: EGLN1; EPAS1; HIF; PHD2; high-altitude adaptation.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Aldenderfer M., Peopling the Tibetan plateau: Insights from archaeology. High Alt. Med. Biol. 12, 141–147 (2011). - PubMed
-
- Zhang X. L., et al. , The earliest human occupation of the high-altitude Tibetan Plateau 40 thousand to 30 thousand years ago. Science 362, 1049–1051 (2018). - PubMed
-
- Beall C. M., Adaptation to high altitude: Phenotypes and genotypes. Annu. Rev. Anthropol. 43, 251–272 (2014).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

