Liquid-liquid phase separation of type II diabetes-associated IAPP initiates hydrogelation and aggregation
- PMID: 32414928
- PMCID: PMC7275713
- DOI: 10.1073/pnas.1916716117
Liquid-liquid phase separation of type II diabetes-associated IAPP initiates hydrogelation and aggregation
Abstract
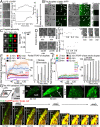
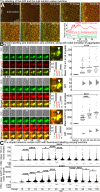
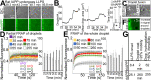
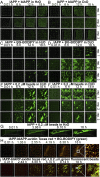
Amyloidoses (misfolded polypeptide accumulation) are among the most debilitating diseases our aging societies face. Amyloidogenesis can be catalyzed by hydrophobic-hydrophilic interfaces (e.g., air-water interface in vitro [AWI]). We recently demonstrated hydrogelation of the amyloidogenic type II diabetes-associated islet amyloid polypeptide (IAPP), a hydrophobic-hydrophilic interface-dependent process with complex kinetics. We demonstrate that human IAPP undergoes AWI-catalyzed liquid-liquid phase separation (LLPS), which initiates hydrogelation and aggregation. Insulin modulates these processes but does not prevent them. Using nonamyloidogenic rat IAPP, we show that, whereas LLPS does not require the amyloidogenic sequence, hydrogelation and aggregation do. Interestingly, both insulin and rat sequence delayed IAPP LLPS, which may reflect physiology. By developing an experimental setup and analysis tools, we show that, within the whole system (beyond the droplet stage), macroscopic interconnected aggregate clusters form, grow, fuse, and evolve via internal rearrangement, leading to overall hydrogelation. As the AWI-adsorbed gelled layer matures, its microviscosity increases. LLPS-driven aggregation may be a common amyloid feature and integral to pathology.
Keywords: IAPP; aggregation; hydrogelation; insulin; liquid–liquid phase separation.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
-
- Stefani M., Dobson C. M., Protein aggregation and aggregate toxicity: New insights into protein folding, misfolding diseases and biological evolution. J. Mol. Med. (Berl.) 81, 678–699 (2003). - PubMed
-
- Westermark P., Andersson A., Westermark G. T., Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 91, 795–826 (2011). - PubMed
-
- Jean L., Lee C. F., Lee C., Shaw M., Vaux D. J., Competing discrete interfacial effects are critical for amyloidogenesis. FASEB J. 24, 309–317 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

