Application of combinatorial optimization strategies in synthetic biology
- PMID: 32415065
- PMCID: PMC7229011
- DOI: 10.1038/s41467-020-16175-y
Application of combinatorial optimization strategies in synthetic biology
Abstract
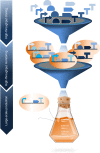
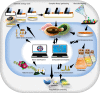
In the first wave of synthetic biology, genetic elements, combined into simple circuits, are used to control individual cellular functions. In the second wave of synthetic biology, the simple circuits, combined into complex circuits, form systems-level functions. However, efforts to construct complex circuits are often impeded by our limited knowledge of the optimal combination of individual circuits. For example, a fundamental question in most metabolic engineering projects is the optimal level of enzymes for maximizing the output. To address this point, combinatorial optimization approaches have been established, allowing automatic optimization without prior knowledge of the best combination of expression levels of individual genes. This review focuses on current combinatorial optimization methods and emerging technologies facilitating their applications.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

