Single cell transcriptomics identifies stem cell-derived graft composition in a model of Parkinson's disease
- PMID: 32415072
- PMCID: PMC7229159
- DOI: 10.1038/s41467-020-16225-5
Single cell transcriptomics identifies stem cell-derived graft composition in a model of Parkinson's disease
Erratum in
-
Author Correction: Single cell transcriptomics identifies stem cell-derived graft composition in a model of Parkinson's disease.Nat Commun. 2020 Jul 15;11(1):3630. doi: 10.1038/s41467-020-17421-z. Nat Commun. 2020. PMID: 32669619 Free PMC article.
Abstract
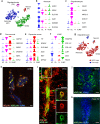
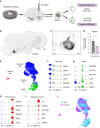
Cell replacement is a long-standing and realistic goal for the treatment of Parkinson's disease (PD). Cells for transplantation can be obtained from fetal brain tissue or from stem cells. However, after transplantation, dopamine (DA) neurons are seen to be a minor component of grafts, and it has remained difficult to determine the identity of other cell types. Here, we report analysis by single-cell RNA sequencing (scRNA-seq) combined with comprehensive histological analyses to characterize intracerebral grafts from human embryonic stem cells (hESCs) and fetal tissue after functional maturation in a pre-clinical rat PD model. We show that neurons and astrocytes are major components in both fetal and stem cell-derived grafts. Additionally, we identify a cell type closely resembling a class of recently identified perivascular-like cells in stem cell-derived grafts. Thus, this study uncovers previously unknown cellular diversity in a clinically relevant cell replacement PD model.
Conflict of interest statement
M.P. is the owner of Parmar Cells AB and co-inventor of the U.S. patent application 15/093,927 owned by Biolamina AB and EP17181588 owned by Miltenyi Biotec. All other authors declare no competing interests.
Figures


References
-
- Cooper O, et al. Differentiation of human ES and Parkinson’s disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol. Cell Neurosci. 2010;45:258–266. doi: 10.1016/j.mcn.2010.06.017. - DOI - PMC - PubMed
-
- Barker RA, Parmar M, Studer L, Takahashi J. Human trials of stem cell-derived dopamine neurons for Parkinson’s disease: dawn of a new era. Stem Cell. 2017;21:569–573. - PubMed
-
- Barker, R. A. TRANSEURO consortium. Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat. Med.25, 1045–1053 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

