Clinically Applicable AI System for Accurate Diagnosis, Quantitative Measurements, and Prognosis of COVID-19 Pneumonia Using Computed Tomography
- PMID: 32416069
- PMCID: PMC7196900
- DOI: 10.1016/j.cell.2020.04.045
Clinically Applicable AI System for Accurate Diagnosis, Quantitative Measurements, and Prognosis of COVID-19 Pneumonia Using Computed Tomography
Erratum in
-
Clinically Applicable AI System for Accurate Diagnosis, Quantitative Measurements, and Prognosis of COVID-19 Pneumonia Using Computed Tomography.Cell. 2020 Sep 3;182(5):1360. doi: 10.1016/j.cell.2020.08.029. Cell. 2020. PMID: 32888496 Free PMC article. No abstract available.
Abstract
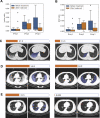
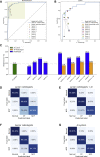
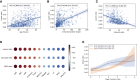
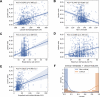
Many COVID-19 patients infected by SARS-CoV-2 virus develop pneumonia (called novel coronavirus pneumonia, NCP) and rapidly progress to respiratory failure. However, rapid diagnosis and identification of high-risk patients for early intervention are challenging. Using a large computed tomography (CT) database from 3,777 patients, we developed an AI system that can diagnose NCP and differentiate it from other common pneumonia and normal controls. The AI system can assist radiologists and physicians in performing a quick diagnosis especially when the health system is overloaded. Significantly, our AI system identified important clinical markers that correlated with the NCP lesion properties. Together with the clinical data, our AI system was able to provide accurate clinical prognosis that can aid clinicians to consider appropriate early clinical management and allocate resources appropriately. We have made this AI system available globally to assist the clinicians to combat COVID-19.
Keywords: AI; COVID-19; SARS-CoV-2; automated diagnosis; computed tomography; deep learning; pneumonia; prognosis analysis.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
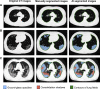

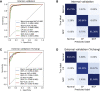
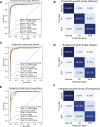
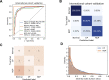


References
-
- Badrinarayanan V., Kendall A., Cipolla R. Segnet: A deep convolutional encoder-decoder architecture for image segmentation. IEEE Trans. Pattern Anal. Mach. Intell. 2017;39:2481–2495. - PubMed
-
- Chen L.-C., Papandreou G., Schroff F., Adam H. Rethinking atrous convolution for semantic image segmentation. arXiv:170605587. 2017
-
- Chen A., Karwoski R.A., Gierada D.S., Bartholmai B.J., Koo C.W. Quantitative CT Analysis of Diffuse Lung Disease. Radiographics. 2020;40:28–43. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

