Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19
- PMID: 32416070
- PMCID: PMC7227586
- DOI: 10.1016/j.cell.2020.04.026
Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19
Abstract
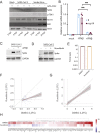
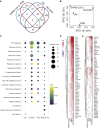
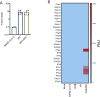
Viral pandemics, such as the one caused by SARS-CoV-2, pose an imminent threat to humanity. Because of its recent emergence, there is a paucity of information regarding viral behavior and host response following SARS-CoV-2 infection. Here we offer an in-depth analysis of the transcriptional response to SARS-CoV-2 compared with other respiratory viruses. Cell and animal models of SARS-CoV-2 infection, in addition to transcriptional and serum profiling of COVID-19 patients, consistently revealed a unique and inappropriate inflammatory response. This response is defined by low levels of type I and III interferons juxtaposed to elevated chemokines and high expression of IL-6. We propose that reduced innate antiviral defenses coupled with exuberant inflammatory cytokine production are the defining and driving features of COVID-19.
Keywords: COVID-19; Coronavirus; IL6; SARS-CoV-2; chemokines; ferret; interferon; transcriptomics; virus-host interactions.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


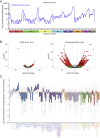
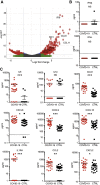

References
-
- Blanco-Melo D., Nilsson-Payant B.E., Uhl S., Escudero-Pèrez B., Olschewski S., Thibault P., Panis M., Rosenthal M., Muñoz-Fontela C., Lee B. An inability to maintain the ribonucleoprotein genomic structure is responsible for host detection of negative-sense RNA viruses. bioRxiv. 2020 doi: 10.1101/2020.2003.2012.989319. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

