Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study
- PMID: 32416072
- PMCID: PMC7523268
- DOI: 10.1016/S1470-2045(20)30157-1
Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study
Erratum in
-
Correction to Lancet Oncol 2020; 21: 796-807.Lancet Oncol. 2020 Oct;21(10):e462. doi: 10.1016/S1470-2045(20)30547-7. Lancet Oncol. 2020. PMID: 33002440 No abstract available.
-
Correction to Lancet Oncol 2020; 21: 796-807.Lancet Oncol. 2024 Feb;25(2):e61. doi: 10.1016/S1470-2045(24)00013-5. Lancet Oncol. 2024. PMID: 38301702 No abstract available.
Abstract
Background: Isocitrate dehydrogenase 1 (IDH1) mutations occur in approximately 13% of patients with intrahepatic cholangiocarcinoma, a relatively uncommon cancer with a poor clinical outcome. The aim of this international phase 3 study was to assess the efficacy and safety of ivosidenib (AG-120)-a small-molecule targeted inhibitor of mutated IDH1-in patients with previously treated IDH1-mutant cholangiocarcinoma.
Methods: This multicentre, randomised, double-blind, placebo-controlled, phase 3 study included patients from 49 hospitals in six countries aged at least 18 years with histologically confirmed, advanced, IDH1-mutant cholangiocarcinoma who had progressed on previous therapy, and had up to two previous treatment regimens for advanced disease, an Eastern Cooperative Oncology Group performance status score of 0 or 1, and a measurable lesion as defined by Response Evaluation Criteria in Solid Tumors version 1.1. Patients were randomly assigned (2:1) with a block size of 6 and stratified by number of previous systemic treatment regimens for advanced disease to oral ivosidenib 500 mg or matched placebo once daily in continuous 28-day cycles, by means of an interactive web-based response system. Placebo to ivosidenib crossover was permitted on radiological progression per investigator assessment. The primary endpoint was progression-free survival by independent central review. The intention-to-treat population was used for the primary efficacy analyses. Safety was assessed in all patients who had received at least one dose of ivosidenib or placebo. Enrolment is complete; this study is registered with ClinicalTrials.gov, NCT02989857.
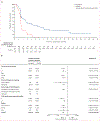
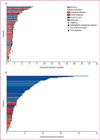
Findings: Between Feb 20, 2017, and Jan 31, 2019, 230 patients were assessed for eligibility, and as of the Jan 31, 2019 data cutoff date, 185 patients were randomly assigned to ivosidenib (n=124) or placebo (n=61). Median follow-up for progression-free survival was 6·9 months (IQR 2·8-10·9). Progression-free survival was significantly improved with ivosidenib compared with placebo (median 2·7 months [95% CI 1·6-4·2] vs 1·4 months [1·4-1·6]; hazard ratio 0·37; 95% CI 0·25-0·54; one-sided p<0·0001). The most common grade 3 or worse adverse event in both treatment groups was ascites (four [7%] of 59 patients receiving placebo and nine [7%] of 121 patients receiving ivosidenib). Serious adverse events were reported in 36 (30%) of 121 patients receiving ivosidenib and 13 (22%) of 59 patients receiving placebo. There were no treatment-related deaths.
Interpretation: Progression-free survival was significantly improved with ivosidenib compared with placebo, and ivosidenib was well tolerated. This study shows the clinical benefit of targeting IDH1 mutations in advanced, IDH1-mutant cholangiocarcinoma.
Funding: Agios Pharmaceuticals.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

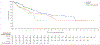
Comment in
-
Towards greater clarity in the treatment of cholangiocarcinoma.Lancet Oncol. 2020 Jun;21(6):738-739. doi: 10.1016/S1470-2045(20)30214-X. Epub 2020 May 13. Lancet Oncol. 2020. PMID: 32416071 No abstract available.
-
Ivosidenib for advanced IDH1-mutant cholangiocarcinoma.Lancet Oncol. 2020 Aug;21(8):e370. doi: 10.1016/S1470-2045(20)30343-0. Lancet Oncol. 2020. PMID: 32758465 No abstract available.
-
Ivosidenib for advanced IDH1-mutant cholangiocarcinoma - Authors' reply.Lancet Oncol. 2020 Aug;21(8):e371. doi: 10.1016/S1470-2045(20)30394-6. Lancet Oncol. 2020. PMID: 32758466 No abstract available.
References
-
- Kipp BR, Voss JS, Kerr SE, et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol 2012; 43: 1552–58. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

