Dose-finding study of a 90-day contraceptive vaginal ring releasing estradiol and segesterone acetate
- PMID: 32416145
- PMCID: PMC7483386
- DOI: 10.1016/j.contraception.2020.05.004
Dose-finding study of a 90-day contraceptive vaginal ring releasing estradiol and segesterone acetate
Erratum in
-
Corrigendum to "Dose-finding study of a 90-day contraceptive vaginal ring releasing estradiol and segesterone acetate" [Contraception 102(3) (2020) 168-173].Contraception. 2020 Dec;102(6):433. doi: 10.1016/j.contraception.2020.09.004. Epub 2020 Oct 24. Contraception. 2020. PMID: 33289653 No abstract available.
Abstract
Objective: To evaluate serum estradiol (E2) concentrations during use of 90-day contraceptive vaginal rings releasing E2 75, 100, or 200 mcg/day and segesterone acetate (SA) 200 mcg/day to identify a dose that avoids hypoestrogenism.
Study design: We conducted a multicenter dose-finding study in healthy, reproductive-aged women with regular cycles with sequential enrollment to increasing E2 dose groups. We evaluated serum E2 concentrations twice weekly for the primary outcome of median E2 concentrations throughout initial 30-day use (target ≥40 pg/mL). In an optional 2-cycle extension substudy, we randomized participants to 2- or 4-day ring-free intervals per 30-day cycle to evaluate bleeding and spotting based on daily diary information.
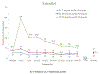
Results: Sixty-five participants enrolled in E2 75 (n = 22), 100 (n = 21), and 200 (n = 22) mcg/day groups; 35 participated in the substudy. Median serum E2 concentrations in 75 and 100 mcg/day groups were <40 pg/mL. In the 200 mcg/day group, median E2 concentrations peaked on days 4-5 of CVR use at 194 pg/mL (range 114-312 pg/mL) and remained >40 pg/mL throughout 30 days; E2 concentrations were 37 pg/mL (range 28-62 pg/mL) on days 88-90 (n = 11). Among the E2 200 mcg/day substudy participants, all had withdrawal bleeding following ring removal. The 2-day ring-free interval group reported zero median unscheduled bleeding and two (range 0-16) and three (range 0-19) unscheduled spotting days in extension cycles 1 and 2, respectively. The 4-day ring-free interval group reported zero median unscheduled bleeding or spotting days.
Conclusions: Estradiol concentrations with rings releasing E2 200 mcg/day and SA 200 mcg/day avoid hypoestrogenism over 30-day use.
Implications: A 90-day contraceptive vaginal ring releasing estradiol 200 mcg/day and segesterone acetate 200 mcg/day achieves estradiol concentrations that should avoid hypoestrogenism and effectively suppresses ovulation.
Keywords: Clinical trial; Contraception; Estradiol; Nestorone®; Segesterone acetate; Vaginal ring.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Dr. Chen and Dr. Barnhart have no financial conflicts of interest to disclose.
Figures

Similar articles
-
Continuous dosing of a novel contraceptive vaginal ring releasing Nestorone® and estradiol: pharmacokinetics from a dose-finding study.Contraception. 2018 May;97(5):422-427. doi: 10.1016/j.contraception.2018.01.012. Epub 2018 Feb 2. Contraception. 2018. PMID: 29409834 Free PMC article. Clinical Trial.
-
A comparative study of two contraceptive vaginal rings releasing norethindrone acetate and differing doses of ethinyl estradiol.Contraception. 1999 May;59(5):305-10. doi: 10.1016/s0010-7824(99)00036-0. Contraception. 1999. PMID: 10494484 Clinical Trial.
-
The use of serum segesterone acetate levels to assess adherence of trial participants with a contraceptive vaginal ring.Contraception. 2022 Apr;108:61-64. doi: 10.1016/j.contraception.2021.12.004. Epub 2021 Dec 28. Contraception. 2022. PMID: 34971614 Clinical Trial.
-
A technology evaluation of Annovera: a segesterone acetate and ethinyl estradiol vaginal ring used to prevent pregnancy for up to one year.Expert Opin Drug Deliv. 2020 Jun;17(6):743-752. doi: 10.1080/17425247.2020.1764529. Epub 2020 May 15. Expert Opin Drug Deliv. 2020. PMID: 32410464 Review.
-
Comprehensive overview of the recently FDA-approved contraceptive vaginal ring releasing segesterone acetate and ethinylestradiol: A new year-long, patient controlled, reversible birth control method.Expert Rev Clin Pharmacol. 2019 Oct;12(10):953-963. doi: 10.1080/17512433.2019.1669448. Epub 2019 Oct 1. Expert Rev Clin Pharmacol. 2019. PMID: 31526281 Review.
Cited by
-
Simultaneous assay of segesterone acetate (Nestorone®), estradiol, progesterone, and estrone in human serum by LC-MS/MS.Contraception. 2020 Nov;102(5):361-367. doi: 10.1016/j.contraception.2020.08.006. Epub 2020 Aug 21. Contraception. 2020. PMID: 32828731 Free PMC article.
-
Participant experiences with a multipurpose vaginal ring for HIV and pregnancy prevention during a phase 1 clinical trial: learning from users to improve acceptability.Front Reprod Health. 2023 Jul 6;5:1147628. doi: 10.3389/frph.2023.1147628. eCollection 2023. Front Reprod Health. 2023. PMID: 37484873 Free PMC article.
-
Vaginal ring acceptability: A systematic review and meta-analysis of vaginal ring experiences from around the world.Contraception. 2022 Feb;106:16-33. doi: 10.1016/j.contraception.2021.10.001. Epub 2021 Oct 10. Contraception. 2022. PMID: 34644609 Free PMC article.
References
-
- Sitruk-Ware R, Small M, Kumar N, Tsong YY, Sundaram K, Jackanicz T. Nestorone®: clinical applications for contraception and HRT. Steroids 2003;68:907–13. - PubMed
-
- Brache V, Mishell DR, Lahteenmaki P, Alvarez F, Elomaa K, Jackanicz T, et al. Ovarian function during use of vaginal rings delivering three different doses of Nestorone. Contraception 2001;63:257–61. - PubMed
-
- Archer DF, Merkatz RB, Bahamondes L, Westhoff CL, Darney P, Apter D, et al. Efficacy of the 1-year (13-cycle) segesterone acetate and ethinylestradiol contraceptive vaginal system: results of two multicentre, open-label, single-arm, phase 3 trials. Lancet Glob Health 2019;7:e1054–e1064. - PMC - PubMed

