Alanine Aminotransferase and Gamma-Glutamyl Transpeptidase Predict Histologic Improvement in Pediatric Nonalcoholic Steatohepatitis
- PMID: 32416645
- PMCID: PMC7669708
- DOI: 10.1002/hep.31317
Alanine Aminotransferase and Gamma-Glutamyl Transpeptidase Predict Histologic Improvement in Pediatric Nonalcoholic Steatohepatitis
Abstract
Background and aims: Predictive, noninvasive tools are needed to monitor key features of nonalcoholic fatty liver disease (NAFLD) in children that relate to improvement in liver histology. The purpose of this study was to evaluate the relationship between liver chemistries and liver histology using data from the CyNCh (Cysteamine Bitartrate Delayed-Release for the Treatment of NAFLD in Children) clinical trial.
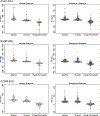
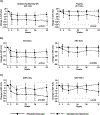
Approach and results: This study included 146 children. Improvement in liver histology, defined as decrease in nonalcoholic fatty liver disease (NAFLD) Activity Score ≥2 points without worsening of fibrosis, occurred in 43 participants (30%). There were 46 participants with borderline zone 1 nonalcoholic steatohepatitis (NASH) at baseline, with resolution in 28% (12 of 46). Multivariate models were constructed using baseline and change in alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT) at 52 weeks, for improvement in (1) liver histology primary outcome, (2) borderline zone 1 NASH, and (3) fibrosis. For improvement in histology, the model (P < 0.0001) retained baseline and change in GGT (area under the receiver operating characteristic [AUROC], 0.79; 95% confidence interval [CI], 0.71-0.87). For borderline zone 1 NASH, the model (P = 0.0004) retained baseline and change in ALT (AUROC, 0.80; 95% CI, 0.67-0.93). For fibrosis, the model (P < 0.001) retained baseline and change in ALT (AUROC, 0.80; 95% CI, 0.67-0.93). Additional clinical parameters were added to the models using Akaike's information criterion selection, and significantly boosted performance: improvement in histology with AUROC of 0.89 (95% CI, 0.82-0.95), borderline zone 1 NASH with AUROC of 0.91 (95% CI, 0.83-0.99), and fibrosis with AUROC of 0.89 (95% CI, 0.82-0.94). Models were validated using data from the TONIC (Treatment of Nonalcoholic Fatty Liver Disease in Children) trial.
Conclusions: In children with NAFLD, dynamic changes in serum ALT and GGT are associated with change in liver histology and appear to be powerful indicators of histological response.
© 2020 by the American Association for the Study of Liver Diseases.
References
-
- Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–93. - PubMed
-
- Fernandes DM, Pantangi V, Azam M, Salomao M, Iuga AC, Lefkowitch JH, et al. Pediatric Nonalcoholic Fatty Liver Disease in New York City: An Autopsy Study. The Journal of pediatrics. 2018;200:174–80. - PubMed
-
- Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). Journal of pediatric gastroenterology and nutrition. 2017;64(2):319–34. - PMC - PubMed
-
- Doycheva I, Issa D, Watt KD, Lopez R, Rifai G, Alkhouri N. Nonalcoholic Steatohepatitis is the Most Rapidly Increasing Indication for Liver Transplantation in Young Adults in the United States. Journal of clinical gastroenterology. 2018;52(4):339–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK061731/DK/NIDDK NIH HHS/United States
- P30 DK078392/DK/NIDDK NIH HHS/United States
- UL1 TR000006/TR/NCATS NIH HHS/United States
- U01 DK061728/DK/NIDDK NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- U01 DK061734/DK/NIDDK NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- U01 DK061737/DK/NIDDK NIH HHS/United States
- U01 DK061713/DK/NIDDK NIH HHS/United States
- U01 DK061732/DK/NIDDK NIH HHS/United States
- P30 DK063720/DK/NIDDK NIH HHS/United States
- UL1 TR000150/TR/NCATS NIH HHS/United States
- U01 DK061718/DK/NIDDK NIH HHS/United States
- UL1 TR001442/TR/NCATS NIH HHS/United States
- M01 RR001271/RR/NCRR NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- U01 DK061730/DK/NIDDK NIH HHS/United States
- U24 DK061730/DK/NIDDK NIH HHS/United States
- P30 DK098722/DK/NIDDK NIH HHS/United States
- U01 DK061738/DK/NIDDK NIH HHS/United States
- UL1 TR000424/TR/NCATS NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- UL1 TR000100/TR/NCATS NIH HHS/United States