Enhanced Human Immunodeficiency Virus-1 Replication in CD4+ T Cells Derived From Individuals With Latent Mycobacterium tuberculosis Infection
- PMID: 32417884
- PMCID: PMC7529013
- DOI: 10.1093/infdis/jiaa257
Enhanced Human Immunodeficiency Virus-1 Replication in CD4+ T Cells Derived From Individuals With Latent Mycobacterium tuberculosis Infection
Abstract
Background: Mycobacterium tuberculosis (Mtb) and human immunodeficiency virus (HIV) coinfection increases mortality, accelerates progression to acquired immune deficiency syndrome, and exacerbates tuberculosis disease. However, the impact of pre-existing Mtb infection on subsequent HIV infection has not been fully explored. We hypothesized that Mtb infection creates an immunological environment that influences the course of HIV infection, and we investigated whether pre-existing Mtb infection impacts the susceptibility of CD4+ T cells to HIV-1 infection.
Methods: Plasma and blood CD4+ T cells isolated from HIV-negative individuals across the Mtb infection spectrum and non-Mtb-infected control individuals were analyzed for inflammation markers and T-cell phenotypes. CD4+ T cells were infected with HIV-1 in vitro and were monitored for viral replication.
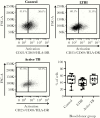
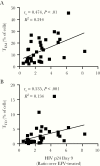
Results: We observed differences in proinflammatory cytokines and the relative proportion of memory T-cell subsets depending on Mtb infection status. CD4+ T cells derived from individuals with latent Mtb infection supported more efficient HIV-1 transcription, release, and replication. Enhanced HIV-1 replication correlated with higher percentages of CD4+ TEM and TTD cells.
Conclusions: Pre-existing Mtb infection creates an immunological environment that reflects Mtb infection status and influences the susceptibility of CD4+ T cells to HIV-1 replication. These findings provide cellular and molecular insights into how pre-existing Mtb infection influences HIV-1 pathogenesis.
Keywords: CD4 T cells; HIV replication; TB immune responses; TB-HIV coinfection; latent tuberculosis infection (LTBI).
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures




References
-
- World Health Organization. Global Tuberculosis Report 2019. Geneva: World Health Organization; 2019.
-
- Joint United Nations Programme on HIV and AIDS (UNAIDS). UNAIDS Data 2019. Geneva, Switzerland: UNAIDS; 2019:16–9. Available at: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_... . Accessed 07 November 2019.
-
- World Health Organization. Global Tuberculosis Control: Epidemiology, Strategy, Financing: WHO Report 2009. Geneva: World Health Organization; 2009.
-
- Whalen C, Horsburgh CR, Hom D, Lahart C, Simberkoff M, Ellner J. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am J Respir Crit Care Med 1995; 151:129–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

