The epidemiology of invasive meningococcal disease and the utility of vaccination in Malta
- PMID: 32418063
- PMCID: PMC7229431
- DOI: 10.1007/s10096-020-03914-8
The epidemiology of invasive meningococcal disease and the utility of vaccination in Malta
Abstract
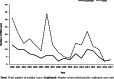
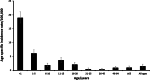
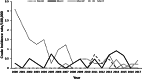
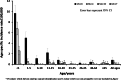
Invasive meningococcal disease (IMD) is a vaccine-preventable devastating infection that mainly affects infants, children and adolescents. We describe the population epidemiology of IMD in Malta in order to assess the potential utility of a meningococcal vaccination programme. All cases of microbiologically confirmed IMD in the Maltese population from 2000 to 2017 were analysed to quantify the overall and capsular-specific disease burden. Mean overall crude and age-specific meningococcal incidence rates were calculated to identify the target age groups that would benefit from vaccination. Over the 18-year study period, 111 out of the 245 eligible notified cases were confirmed microbiologically of which 70.3% had septicaemia, 21.6% had meningitis, and 6.3% had both. The mean overall crude incidence rate was 1.49/100,000 population with an overall case fatality rate of 12.6%. Meningococcal capsular groups (Men) B followed by C were the most prevalent with W and Y appearing over the last 6 years. Infants had the highest meningococcal incidence rate of 18.9/100,000 followed by 6.1/100,000 in 1-5 year olds and 3.6/100,000 in 11-15 year old adolescents. The introduction of MenACWY and MenB vaccines on the national immunization schedule in Malta would be expected to reduce the disease burden of meningococcal disease in children and adolescents in Malta.
Keywords: Conjugate vaccination; Epidemiology; Malta; MenB vaccination; Meningococcus.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- ECDC: European Centre for Disease Prevention and Control. Surveillance Report. Invasive meningococcal disease. Annual Epidemiological Report for 2017. Stockholm 2019. Available from https://ecdc.europa.eu/sites/portal/ files/documents/AER_for_2017-invasive-meningococcal-disease.pdf. Accessed 20th April, 2020
-
- CDC: Centers for Disease Control and Prevention. Active Bacterial Core surveillance (ABCs). Surveillance reports: Neisseria meningitidis, 1997–2017. Available from http://www.cdc.gov/abcs/reports-findings/surv-reports.html. Accessed 20th April, 2020
-
- Wang B, Santoreneos R, Giles L, Haji Ali Afzali H, Marshall H. Case fatality rates of invasive meningococcal disease by serogroup and age: a systematic review and meta-analysis. Vaccine. 2019;37(21):2768–2782. - PubMed
-
- Sadarangani M, Scheifele DW, Halperin SA, Vaudry W, Le Saux N, Tsang R, Bettinger JA. Investigators of the Canadian Immunization Monitoring Program, ACTive (IMPACT). Outcomes of invasive meningococcal disease in adults and children in Canada between 2002 and 2011: a prospective cohort study. Clin Infect Dis. 2015;60(8):e27–e35. - PubMed
-
- Davis KL, Misurski D, Miller J, Karve S. Cost impact of complications in meningococcal disease: evidence from a United States managed care population. Hum Vaccin. 2011;7(4):458–465. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

