Deletion of JNK Enhances Senescence in Joint Tissues and Increases the Severity of Age-Related Osteoarthritis in Mice
- PMID: 32418287
- PMCID: PMC7669715
- DOI: 10.1002/art.41312
Deletion of JNK Enhances Senescence in Joint Tissues and Increases the Severity of Age-Related Osteoarthritis in Mice
Abstract
Objective: To determine the role of JNK signaling in the development of osteoarthritis (OA) induced by joint injury or aging in mice.
Methods: In the joint injury model, 12-week-old wild-type control, JNK1-/- , JNK2-/- , and JNK1fl/fl JNK2-/- aggecan-CreERT 2 double-knockout mice were subjected to destabilization of the medial meniscus (DMM) (n = 15 mice per group) or sham surgery (n = 9-10 mice per group), and OA was evaluated 8 weeks later. In the aging experiment, wild-type control, JNK1-/- , and JNK2-/- mice (n = 15 per group) were evaluated at 18 months of age. Mouse knee joints were evaluated by scoring articular cartilage structure, toluidine blue staining, osteophytes, and synovial hyperplasia, by histomorphometric analysis, and by immunostaining for the senescence marker p16INK 4a . Production of matrix metalloproteinase 13 (MMP-13) in cartilage explants in response to fibronectin fragments was measured by enzyme-linked immunosorbent assay.
Results: There were no differences after DMM surgery between the wild-type and the JNK-knockout mouse groups in articular cartilage structure, toluidine blue, or osteophyte scores or in MMP-13 production in explants. All 3 knockout mouse groups had increased subchondral bone thickness and area of cartilage necrosis compared to wild-type mice. Aged JNK-knockout mice had significantly worse articular cartilage structure scores compared to the aged wild-type control mice (mean ± SD 52 ± 24 in JNK1-/- mice and 60 ± 25 in JNK2-/- mice versus 32 ± 18 in controls; P = 0.02 and P = 0.004, respectively). JNK1-/- mice also had higher osteophyte scores. Deletion of JNK resulted in increased expression of p16INK 4a in the synovium and cartilage in older mice.
Conclusion: JNK1 and JNK2 are not required for the development of OA in the mouse DMM model. Deletion of JNK1 or JNK2 is associated with more severe age-related OA and increased cell senescence, suggesting that JNK may act as a negative regulator of senescence in the joint.
© 2020, American College of Rheumatology.
Conflict of interest statement
Conflict of interest
Dr. Loeser has received consulting fees from Unity Biotechnology (less than $2000).
Figures

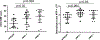
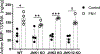

Comment in
-
JNK Pathway as a Target for Osteoarthritis: Comment on the Article by Loeser et al.Arthritis Rheumatol. 2020 Dec;72(12):2162. doi: 10.1002/art.41432. Epub 2020 Oct 6. Arthritis Rheumatol. 2020. PMID: 32869546 No abstract available.
-
Reply.Arthritis Rheumatol. 2020 Dec;72(12):2162-2163. doi: 10.1002/art.41431. Epub 2020 Sep 30. Arthritis Rheumatol. 2020. PMID: 33459503 Free PMC article. No abstract available.
References
-
- Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22 (4):667–76. - PubMed
-
- Clancy R, Rediske J, Koehne C, Stoyanovsky D, Amin A, Attur M, et al. Activation of stress-activated protein kinase in osteoarthritic cartilage: evidence for nitric oxide dependence. Osteoarthritis Cartilage. 2001;9 (4):294–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

