Self-assembly of multiscale anisotropic hydrogels through interfacial polyionic complexation
- PMID: 32418322
- PMCID: PMC11540064
- DOI: 10.1002/jbm.a.37001
Self-assembly of multiscale anisotropic hydrogels through interfacial polyionic complexation
Abstract
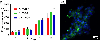
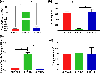
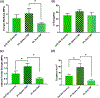
Polysaccharides are explored for various tissue engineering applications due to their inherent cytocompatibility and ability to form bulk hydrogels. However, bulk hydrogels offer poor control over their microarchitecture and multiscale hierarchy, parameters important to recreate extracellular matrix-mimetic microenvironment. Here, we developed a versatile platform technology to self-assemble oppositely charged polysaccharides into multiscale fibrous hydrogels with controlled anisotropic microarchitecture. We employed polyionic complexation through microfluidic flow of positively charged polysaccharide, chitosan, along with one of the three negatively charged polysaccharides: alginate, gellan gum, and kappa carrageenan. These hydrogels were composed of microscale fibers, which in turn were made of submicron fibrils confirming multiscale hierarchy. Fibrous hydrogels showed strong tensile mechanical properties, which were further modulated by encapsulation of shape-specific antioxidant cerium oxide nanoparticles (CNPs). Specifically, hydrogels with chitosan and gellan gum showed more than eight times higher tensile strength compared to the other two pairs. Incorporation of sphere-shaped cerium oxide nanoparticles in chitosan and gellan gum further reinforced fibrous hydrogels and increased their tensile strength by 40%. Altogether, our automated hydrogel fabrication platform allows fabrication of bioinspired biomaterials with scope for one-step encapsulation of small molecules and nanoparticles without chemical modification or use of chemical crosslinkers.
Keywords: alginate; automated collector; cerium oxide nanoparticles; chitosan; fibrous hydrogels; gellan gum; interfacial polyionic complexation; kappa carrageenan; polysaccharides.
© 2020 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Figures
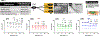



References
-
- Abbah SA, Liu J, Lam RWM, Goh JCH, & Wong HK (2012). In vivo bioactivity of rhBMP-2 delivered with novel polyelectrolyte complexation shells assembled on an alginate microbead core template. Journal of Controlled Release, 162(2), 364–372. - PubMed
-
- Amaike M, Senoo Y, & Yamamoto H (1998). Sphere, honeycomb, regularly spaced droplet and fiber structures of polyion complexes of chitosan and gellan. Macromolecular Rapid Communications, 19(6), 287–289.
-
- Arenales-Sierra IM, Lobato-Calleros C, Vernon-Carter EJ, Hernandez-Rodriguez L, & Alvarez-Ramirez J (2019). Calcium alginate beads loaded with Mg(OH)(2) improve L. casei viability under simulated gastric condition. Lwt-Food Science and Technology, 112.
-
- Bidarra SJ, Barrias CC, & Granja PL (2014). Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomaterialia, 10, 1646–1662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

