A Validated Model for Sudden Cardiac Death Risk Prediction in Pediatric Hypertrophic Cardiomyopathy
- PMID: 32418493
- PMCID: PMC7365676
- DOI: 10.1161/CIRCULATIONAHA.120.047235
A Validated Model for Sudden Cardiac Death Risk Prediction in Pediatric Hypertrophic Cardiomyopathy
Abstract
Background: Hypertrophic cardiomyopathy is the leading cause of sudden cardiac death (SCD) in children and young adults. Our objective was to develop and validate a SCD risk prediction model in pediatric hypertrophic cardiomyopathy to guide SCD prevention strategies.
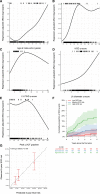
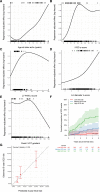
Methods: In an international multicenter observational cohort study, phenotype-positive patients with isolated hypertrophic cardiomyopathy <18 years of age at diagnosis were eligible. The primary outcome variable was the time from diagnosis to a composite of SCD events at 5-year follow-up: SCD, resuscitated sudden cardiac arrest, and aborted SCD, that is, appropriate shock following primary prevention implantable cardioverter defibrillators. Competing risk models with cause-specific hazard regression were used to identify and quantify clinical and genetic factors associated with SCD. The cause-specific regression model was implemented using boosting, and tuned with 10 repeated 4-fold cross-validations. The final model was fitted using all data with the tuned hyperparameter value that maximizes the c-statistic, and its performance was characterized by using the c-statistic for competing risk models. The final model was validated in an independent external cohort (SHaRe [Sarcomeric Human Cardiomyopathy Registry], n=285).
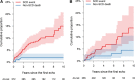
Results: Overall, 572 patients met eligibility criteria with 2855 patient-years of follow-up. The 5-year cumulative proportion of SCD events was 9.1% (14 SCD, 25 resuscitated sudden cardiac arrests, and 14 aborted SCD). Risk predictors included age at diagnosis, documented nonsustained ventricular tachycardia, unexplained syncope, septal diameter z-score, left ventricular posterior wall diameter z score, left atrial diameter z score, peak left ventricular outflow tract gradient, and presence of a pathogenic variant. Unlike in adults, left ventricular outflow tract gradient had an inverse association, and family history of SCD had no association with SCD. Clinical and clinical/genetic models were developed to predict 5-year freedom from SCD. Both models adequately discriminated between patients with and without SCD events with a c-statistic of 0.75 and 0.76, respectively, and demonstrated good agreement between predicted and observed events in the primary and validation cohorts (validation c-statistic 0.71 and 0.72, respectively).
Conclusion: Our study provides a validated SCD risk prediction model with >70% prediction accuracy and incorporates risk factors that are unique to pediatric hypertrophic cardiomyopathy. An individualized risk prediction model has the potential to improve the application of clinical practice guidelines and shared decision making for implantable cardioverter defibrillator insertion. Registration: URL: https://www.clinicaltrials.gov; Unique identifier: NCT0403679.
Trial registration: ClinicalTrials.gov NCT04036799.
Keywords: cardiomyopathies; cardiomyopathy, hypertrophic; death, sudden, heart; defibrillators, implantable; hypertrophy; pediatrics.
Figures
Comment in
-
Response by Mital et al to Letter Regarding Article, "A Validated Model for Sudden Cardiac Death Risk Prediction in Pediatric Hypertrophic Cardiomyopathy".Circulation. 2021 Mar 16;143(11):e788-e789. doi: 10.1161/CIRCULATIONAHA.120.051632. Epub 2021 Mar 15. Circulation. 2021. PMID: 33720772 No abstract available.
-
Letter by Grace et al Regarding Article, "A Validated Model for Sudden Cardiac Death Risk Prediction in Pediatric Hypertrophic Cardiomyopathy".Circulation. 2021 Mar 16;143(11):e786-e787. doi: 10.1161/CIRCULATIONAHA.120.051270. Epub 2021 Mar 15. Circulation. 2021. PMID: 33720776 No abstract available.
References
-
- Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Circulation 199592785–789doi: 10.1161/01.cir.92.4.785 - PubMed
-
- Semsarian C, Ingles J, Maron MS, Maron BJ. New perspectives on the prevalence of hypertrophic cardiomyopathy. J Am Coll Cardiol 2015651249–1254doi: 10.1016/j.jacc.2015.01.019 - PubMed
-
- Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 201158e212–e260doi: 10.1016/j.jacc.2011.06.011 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

