CITED4 Protects Against Adverse Remodeling in Response to Physiological and Pathological Stress
- PMID: 32418505
- PMCID: PMC7725361
- DOI: 10.1161/CIRCRESAHA.119.315881
CITED4 Protects Against Adverse Remodeling in Response to Physiological and Pathological Stress
Abstract
Rationale: Cardiac CITED4 (CBP/p300-interacting transactivators with E [glutamic acid]/D [aspartic acid]-rich-carboxylterminal domain4) is induced by exercise and is sufficient to cause physiological hypertrophy and mitigate adverse ventricular remodeling after ischemic injury. However, the role of endogenous CITED4 in response to physiological or pathological stress is unknown.
Objective: To investigate the role of CITED4 in murine models of exercise and pressure overload.
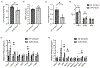
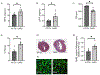
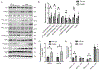
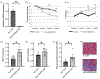
Methods and results: We generated cardiomyocyte-specific CITED4 knockout mice (C4KO) and subjected them to an intensive swim exercise protocol as well as transverse aortic constriction (TAC). Echocardiography, Western blotting, qPCR, immunohistochemistry, immunofluorescence, and transcriptional profiling for mRNA and miRNA (microRNA) expression were performed. Cellular crosstalk was investigated in vitro. CITED4 deletion in cardiomyocytes did not affect baseline cardiac size or function in young adult mice. C4KO mice developed modest cardiac dysfunction and dilation in response to exercise. After TAC, C4KOs developed severe heart failure with left ventricular dilation, impaired cardiomyocyte growth accompanied by reduced mTOR (mammalian target of rapamycin) activity and maladaptive cardiac remodeling with increased apoptosis, autophagy, and impaired mitochondrial signaling. Interstitial fibrosis was markedly increased in C4KO hearts after TAC. RNAseq revealed induction of a profibrotic miRNA network. miR30d was decreased in C4KO hearts after TAC and mediated crosstalk between cardiomyocytes and fibroblasts to modulate fibrosis. miR30d inhibition was sufficient to increase cardiac dysfunction and fibrosis after TAC.
Conclusions: CITED4 protects against pathological cardiac remodeling by regulating mTOR activity and a network of miRNAs mediating cardiomyocyte to fibroblast crosstalk. Our findings highlight the importance of CITED4 in response to both physiological and pathological stimuli.
Keywords: exercise; extracellular matrix; heart failure; signal transduction.
Figures




Comment in
-
A Case for Adaptive Cardiac Hypertrophic Remodeling Is CITED.Circ Res. 2020 Aug 14;127(5):647-650. doi: 10.1161/CIRCRESAHA.120.317623. Epub 2020 Aug 13. Circ Res. 2020. PMID: 32790523 No abstract available.
References
-
- Baggish AL. Mechanisms underlying the cardiac benefits of exercise: Still running in the dark. Trends in cardiovascular medicine. 2015;25:537–9. - PubMed
-
- Bostrom P, Mann N, Wu J, Quintero PA, Plovie ER, kova DP, Gupta RK, Xiao C, MacRae CA, Rosenzweig A and Spiegelman BM. C/EBPbeta Controls Exercise-Induced Cardiac Growth and Protects against Pathological Cardiac Remodeling. Cell. 2010;143:1072–1083 (*co-senior, co-corresponding, equal contributing authors). - PMC - PubMed
-
- Yahata T, de Caestecker MP, Lechleider RJ, Andriole S, Roberts AB, Isselbacher KJ and Shioda T. The MSG1 non-DNA-binding transactivator binds to the p300/CBP coactivators, enhancing their functional link to the Smad transcription factors. The Journal of biological chemistry. 2000;275:8825–34. - PubMed
-
- Shioda T, Lechleider RJ, Dunwoodie SL, Li H, Yahata T, de Caestecker MP, Fenner MH, Roberts AB and Isselbacher KJ. Transcriptional activating activity of Smad4: roles of SMAD hetero-oligomerization and enhancement by an associating transactivator. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9785–90. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

