Role of cilia in the pathogenesis of congenital heart disease
- PMID: 32418658
- PMCID: PMC7906359
- DOI: 10.1016/j.semcdb.2020.04.017
Role of cilia in the pathogenesis of congenital heart disease
Abstract
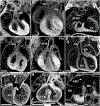
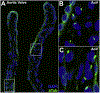
An essential role for cilia in the pathogenesis of congenital heart disease (CHD) has emerged from findings of a large-scale mouse forward genetic screen. High throughput screening with fetal ultrasound imaging followed by whole exome sequencing analysis recovered a preponderance of cilia related genes and cilia transduced cell signaling genes among mutations identified to cause CHD. The perturbation of left-right patterning in CHD pathogenesis is suggested by the association of CHD with heterotaxy, but also by the finding of the co-occurrence of laterality defects with CHD in birth defect registries. Many of the cilia and cilia cell signaling genes recovered were found to be related to Hedgehog signaling. Studies in mice showed cilia transduced hedgehog signaling coordinates left-right patterning with heart looping and differentiation of the heart tube. Cilia transduced Shh signaling also regulates later events in heart development, including outflow tract septation and formation of the atrioventricular septum. More recent work has shown mutations in cilia related genes may also contribute to valve disease that largely manifest in adult life. Overall, these and other findings show cilia play an important role in CHD and also in more common valve diseases.
Keywords: Cilia; Congenital heart disease; Fetal ultrasound imaging; Forward genetics; Mouse models; Mutagenesis.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest statement
The authors declare no conflict of interest.
Figures

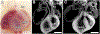

References
-
- Longobardo L, Jain R, Carerj S, Zito C, Khandheria BK, Bicuspid aortic valve: unlocking the morphogenetic puzzle, The American journal of medicine 129(8) (2016) 796–805. - PubMed
-
- Tutar E, Ekici F, Atalay S, Nacar N, The prevalence of bicuspid aortic valve in newborns by echocardiographic screening, American heart journal 150(3) (2005) 513–515. - PubMed
-
- Holst KA, Said SM, Nelson TJ, Cannon BC, Dearani JA, Current interventional and surgical management of congenital heart disease: specific focus on valvular disease and cardiac arrhythmias, Circulation research 120(6) (2017) 1027–1044. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

