"Off-label" use of hydroxychloroquine, azithromycin, lopinavir-ritonavir and chloroquine in COVID-19: A survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance Centers
- PMID: 32418730
- PMCID: PMC7204701
- DOI: 10.1016/j.therap.2020.05.002
"Off-label" use of hydroxychloroquine, azithromycin, lopinavir-ritonavir and chloroquine in COVID-19: A survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance Centers
Abstract
Introduction: COVID-19 is an unprecedented challenge for physicians and scientists. Several publicized drugs are being used with not much evidence of their efficacy such as hydroxychloroquine, azithromycin or lopinavir-ritonavir. Yet, the cardiac safety of these drugs in COVID-19 deserves scrutiny as they are known to foster cardiac adverse ADRs, notably QTc interval prolongation on the electrocardiogram and its arrhythmogenic consequences.
Methods: Since March 27th, 2020, the French Pharmacovigilance Network directed all cardiac adverse drug reactions associated with "off-label" use of hydroxychloroquine, azithromycin and lopinavir-ritonavir in COVID-19 to the Nice Regional Center of Pharmacovigilance. Each Regional Center of Pharmacovigilance first assessed causality of drugs. We performed a specific analysis of these cardiac adverse drug reactions amidst an array of risk factors, reassessed the electrocardiograms and estimated their incidence in coronavirus disease 2019.
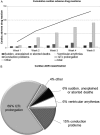
Results: In one month, 120 reports of cardiac adverse drug reactions have been notified, 103 of which associated with hydroxychloroquine alone (86%), or associated with azithromycin (60%). Their estimated incidence is 0.77% to 1.54% of all patients, notwithstanding strong underreporting. Lopinavir-ritonavir came third with 17 reports (14%) and chloroquine fourth with 3 reports (2.5%). There were 8 sudden, unexplained or aborted deaths (7%), 8 ventricular arrhythmias (7%), 90 reports of prolonged QTc (75%) most of them "serious" (64%), 48 of which proved ≥ 500ms, 20 reports of severe conduction disorders (17%) and 5 reports of other cardiac causes (4%). Six reports derived from automedication.
Discussion and conclusion: "Off-label" use of treatments in COVID-19 increases the risk of cardiac ADRs, some of them avoidable. Even if these drugs are perceived as familiar, they are used in patients with added risk factors caused by infection. Precautions should be taken to mitigate the risk, even if they will be proven efficacious.
Keywords: Arrhythmia; Azithromycin; COVID-19; Cardiac adverse effects; Hydroxychloroquine; Lopinavir; QTc prolongation.
Copyright © 2020 Société française de pharmacologie et de thérapeutique. Published by Elsevier Masson SAS. All rights reserved.
Figures


References
-
- Worldometer . 2020. COVID-19 Coronavirus pandemic. http://www.worldometers.info/coronavirus [Accessed May 4, 2020]
-
- Adhanom T. 2020. WHO Director-General's opening remarks at the media briefing on COVID-19. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re... [Accessed May 4, 2020]
-
- Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14(1):72–73. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

