Introducing human adipose-derived mesenchymal stem cells to AvanceⓇ nerve grafts and NeuraGenⓇ nerve guides
- PMID: 32418840
- PMCID: PMC7375015
- DOI: 10.1016/j.bjps.2020.03.012
Introducing human adipose-derived mesenchymal stem cells to AvanceⓇ nerve grafts and NeuraGenⓇ nerve guides
Abstract
Background: When direct nerve coaptation is impossible after peripheral nerve injury, autografts, processed allografts, or conduits are used to bridge the nerve gap. The purpose of this study was to examine if human adipose-derived Mesenchymal Stromal/Stem Cells (MSCs) could be introduced to commercially available nerve graft substitutes and to determine cell distribution and the seeding efficiency of a dynamic seeding strategy.
Methods: MTS assays examined the viability of human MSCs after introduction to the AvanceⓇ Nerve Graft and the NeuraGenⓇ Nerve Guide. MSCs were dynamically seeded on nerve substitutes for either 6, 12, or 24 h. Cell counts, live/dead stains, Hoechst stains, and Scanning Electron Microscopy (SEM) revealed the seeding efficiency and the distribution of MSCs after seeding.
Results: The viability of MSCs was not affected by nerve substitutes. Dynamic seeding led to uniformly distributed MSCs over the surface of both nerve substitutes and revealed MSCs on the inner surface of the NeuraGenⓇ Nerve Guides. The maximal seeding efficiency of NeuraGenⓇ Nerve Guides (94%), obtained after 12 h was significantly higher than that of AvanceⓇ Nerve Grafts (66%) (p = 0.010).
Conclusion: Human MSCs can be dynamically seeded on AvanceⓇ Nerve Grafts and NeuraGenⓇ Nerve Guides. The optimal seeding duration was 12 h. MSCs were distributed in a uniform fashion on exposed surfaces. This study demonstrates that human MSCs can be effectively and efficiently seeded onto commercially available nerve autograft substitutes in a timely fashion and sets the stage for the clinical application of MSC-seeded nerve graft substitutes clinically.
Keywords: Avance(Ⓡ) Nerve Graft; MSCs; NeuraGen(Ⓡ) Nerve Guide; Seeding.
Copyright © 2020. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of Competing Interest The authors have nothing to disclose. This study was funded by the NIH R01, “Bridging the gap: angiogenesis and stem cell seeding of processed nerve allograft” 1 RO1 NS102360-01A1. The Avance(Ⓡ) Nerve Grafts used in this study were provided by AxoGen Inc., Alachua, Florida, USA. The NeuraGen(Ⓡ) Nerve Guides used in this study were provided by Integra LifeSciences Holdings Corporation, Plainsboro, New Jersey, USA.
Figures







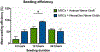
References
-
- Lin MY, Manzano G, Gupta R. Nerve allografts and conduits in peripheral nerve repair. Hand Clin 2013;29:331–48. - PubMed
-
- Brooks DN, Weber RV, Chao JD, Rinker BD, Zoldos J, Robichaux MR, et al. Processed nerve allografts for peripheral nerve reconstruction: A multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurg 2012;32:1–14. - PubMed
-
- Cho MS, Rinker BD, Weber RV, Chao JD, Ingari JV, Brooks D, et al. Functional outcome following nerve repair in the upper extremity using processed nerve allograft. J Hand Surg Am 2012;37:2340–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

