Vasopressin antagonist efficacy and safety in volume-overloaded critically ill patients: a new therapeutic alternative
- PMID: 32419936
- PMCID: PMC7223349
- DOI: 10.1136/ejhpharm-2018-001664
Vasopressin antagonist efficacy and safety in volume-overloaded critically ill patients: a new therapeutic alternative
Abstract
Objectives: To assess tolvaptan's efficacy and safety in critical care patients with volume overload.
Methods: Prospective observational study. Twenty-eight patients in the recovery phase from multiple organ failure and with volume overload refractory to conventional therapy treated with tolvaptan were included.
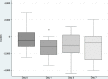
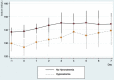
Results: Patients received an initial daily dose of 3.75 (n=1), 7.5 (n=8) and 15 (n=19) mg of tolvaptan. Median treatment duration was 2 days (range: 1 to 12). All patients presented an increase in 24 hours diuresis after the first dose (median increase from baseline (IQR)=1114 (285-1943) mL), with a median net daily fluid loss of 1007 mL (456-2380) mL after 24 hours. High diuretic efficacy (daily fluid loss higher than 0.5 L with tolvaptan first dose) was detected in 18 patients (64.3%). Initial hyponatraemia was present in 16 (57.1%) patients, while overly rapid correction with tolvaptan treatment occurred in two patients without clinical consequences. Two patients presented hypophosphataemia after treatment.
Conclusion: Tolvaptan is an effective therapeutic option in critically ill patients with volume overload refractory to conventional diuretics. Further studies are required to evaluate its safety profile and its effect on short-term outcomes and mortality.
Keywords: critical care; diuretics; edema; fluid balance; tolvaptan.
© European Association of Hospital Pharmacists 2020. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PR and MJB have participated in training courses with funding from the laboratory Otsuka Pharmaceutical Europe.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical