Relationship between sperm morphology and sperm DNA dispersion
- PMID: 32420146
- PMCID: PMC7215007
- DOI: 10.21037/tau.2020.01.31
Relationship between sperm morphology and sperm DNA dispersion
Abstract
Background: The pathogenesis of teratozoospermia (<4% morphologically normal sperm cells) and the relationship between sperm morphological abnormalities and abnormal sperm nuclear DNA fragmentation, which are considered indicators of male fertility, have not been elucidated. Our research was designed to determine the prevalence of different sperm DNA fragmentation (SDF) levels in men with teratozoospermia and to establish a discriminating threshold value for SDF in assessing sperm morphology.
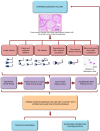
Methods: Basic semen characteristics and detailed sperm morphological analysis (head, neck, midpiece, and tail defects and excess residual cytoplasm) (WHO, 2010), and the nuclear sperm DNA dispersion test were performed on semen samples obtained from 523 men with teratozoospermia (n=296) and those without teratozoospermia (n=227).
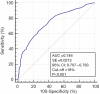
Results: Subjects with abnormal sperm morphology had not only lower results for standard sperm characteristics, including detailed sperm morphological abnormalities, but also a higher proportion of sperm cells with SDF vs. men with normal sperm morphology. Moreover, significantly fewer subjects with low SDF levels (≤15%), more subjects with high SDF levels (>30%) and a higher odds ratio (OR) for having high SDF levels were found in the group of men with teratozoospermia vs. men without teratozoospermia. However, the receiving operating characteristic (ROC) curve analysis indicated that a SDF >18% was a significant negative predictive value to distinguish between men with normal sperm morphology or men with abnormal sperm morphology. The optimal area under the ROC curve (AUC) was 0.746. In the group of men with teratozoospermia, a higher incidence of men with >18% SDF and a higher OR for having >18% SDF were observed. SDF negatively correlated with sperm number, morphologically normal sperm cells, sperm motility and sperm vitality but positively correlated with the teratozoospermia index (TZI) and detailed sperm morphological abnormalities.
Conclusions: The obtained findings demonstrated that: (I) detailed sperm structural defects coexist with abnormal nuclear sperm DNA dispersion, (II) men with teratozoospermia may have a higher risk for sperm DNA damage, (III) the calculated optimal SDF value of 18% measured by the DNA sperm dispersion test is the best criterion to predict normal and abnormal sperm morphology.
Keywords: Male infertility; semen analysis; sperm DNA fragmentation (SDF); sperm morphology.
2020 Translational Andrology and Urology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2020.01.31). The authors have no conflicts of interest to declare.
Figures
References
-
- World Health Organization laboratory manual for the Examination and processing of human semen, 5th edition, WHO Press, World Health Organization, Geneva, Switzerland 2010.
LinkOut - more resources
Full Text Sources
