Do testosterone supplements enhance response to phosphodiesterase 5 inhibitors in men with erectile dysfunction and hypogonadism: a systematic review and meta-analysis
- PMID: 32420164
- PMCID: PMC7215032
- DOI: 10.21037/tau.2020.01.13
Do testosterone supplements enhance response to phosphodiesterase 5 inhibitors in men with erectile dysfunction and hypogonadism: a systematic review and meta-analysis
Abstract
Background: Combining testosterone and phosphodiesterase 5 inhibitors (PDE5-Is) has become increasingly common in the treatment of men with erectile dysfunction (ED) and low testosterone levels, but combination therapy involving PDE5-Is and testosterone is highly debated, with strong reasons for and against argued by the various opinion leaders. PDE5-Is can be given prior to, alongside or after the commencement of any testosterone replacement therapy. Meanwhile, combination of PDE5-Is and testosterone is reported to better increase testosterone levels and thus improve International Index of Erectile Function (IIEF) score in hypogonadal men. The objective of this meta-analysis was to assess whether testosterone therapy (TTh) can possibly enhance the reaction to PDE5-Is in men with ED and hypogonadism.
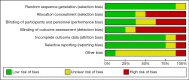
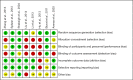
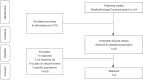
Methods: Relevant studies and available data were extensively collected form Medline, Embase, and Cochrane Library databases until June 2019. We calculated standard mean differences (SMDs) with their 95% confidence intervals (CIs) for IIEF including IIEF-5 and IIEF-EFD. Trial sequential analysis (TSA) was performed to explore whether the sample size of the accumulated evidence is sufficient.
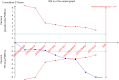
Results: There were 8 studies including 913 patients. The pooled SMD of erectile function (EF) component change was 0.663 [(0.299 to 1.027); P<0.0001], which concluded that combination therapy (TTh plus PDE5-Is) is superior to PDE5-Is monotherapy group. We also conducted a subgroup analysis according to trial follow-up, baseline serum total testosterone, baseline EF score and PDE5-Is type, which may explain for the underlying source of heterogeneity in part. The frequency of adverse events and change in PSA levels did not differ between the 2 groups. None of the patients experienced an increase in the prostate specific antigen (PSA) level above 4 ng/mL. Hematocrit increased significantly more in the testosterone group than in the placebo group but not greater than 0.54.
Conclusions: In summary, the present results confirm that combination therapy is effective and safe. TTh can enhance the reaction to PDE5-Is in men with ED and hypogonadism, but this effect also depends on the specific diagnosis and initial response to PDE5-Is. Most patients with adverse events during treatment are mild, and have a stable overall safety of combination therapy.
Keywords: PDE5 inhibitors; erectile dysfunction (ED); hypogonadism; meta-analysis; testosterone therapy (TTh).
2020 Translational Andrology and Urology. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2020.01.13). The authors have no conflicts of interest to declare.
Figures


References
-
- Impotence. NIH Consens Statement 1992;10:1-33. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
