Nanoformulated Eudragit lopinavir and preliminary release of its loaded suppositories
- PMID: 32420478
- PMCID: PMC7218025
- DOI: 10.1016/j.heliyon.2020.e03890
Nanoformulated Eudragit lopinavir and preliminary release of its loaded suppositories
Abstract
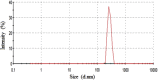
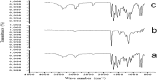
The development of novel paediatrics formulations is critical towards achieving the UNAIDS 90-90-90 targets. According to the latest UNAIDS reports, the availability of antiretrovirals (ARVs) for children has increased significantly, from 49% in 2015 to 53% in 2017. However, this percentage is considerably lower than the 80% for pregnant women that are currently on treatment. Therefore, there is still an urgent need for an alternative child-friendly delivery system. Lopinavir (LPV) is a protease inhibitor first-line HIV treatment drugs but suffers from low aqueous solubility, bitter state, short half-life leading to a limited dissolution and variable bioavailability upon oral administration. This work focused on the fabrication and characterization of a delivery system entailing Eudragit RSPO-LPV nanoparticles loaded suppositories in two different bases to improve the bioavailability and overcome the problem encountered through oral administration emanating from poor solubility. The prepared nanoparticles by nanoprecipitation method were characterized and compounded into suppositories in fattibase and polyethylene glycol (PEG) bases using a melt fusion method. The suppositories were stored at 5 and 25 °C, and were sampled at 0, 4, 8, 12 weeks. The samples were assessed by particle size, entrapment efficiency (EE), zeta potential and polydispersity index (PDI) variations. The preliminary in vitro release studies were analysed by HPLC. The nanoparticles have an average particle size of 191 nm with spherical morphology, entrapment efficiency, polydispersity index and zeta potential of 79.0 ± 0.5%, 0.224, and 25.87 ± 0.41 mV respectively. The surface analysis of the nanoparticles with FTIR, SEM, PXRD and TGA indicated that the drug was truly encapsulated without any interaction. The in vitro release studies showed that a better release was observed in suppositories formulated with PEG than the fattibase by having higher drug concentration released. Hence, this rectal formulation might serve as an alternative for paediatric HIV treatment upon further investigation.
Keywords: Analytical chemistry; Chemistry; Eudragit; HIV paediatric; Health sciences; Melt fusion method; Nanoparticles; Organic chemistry; Pharmaceutical chemistry; Suppositories.
© 2020 The Authors.
Figures







Similar articles
-
Lopinavir-Loaded Self-Nanoemulsifying Drug Delivery System for Enhanced Solubility: Development, Characterisation and Caco-2 Cell Uptake.Curr Drug Deliv. 2023;20(10):1474-1486. doi: 10.2174/1567201819666220817111054. Curr Drug Deliv. 2023. PMID: 35980056
-
Statistical modeling, optimization and characterization of solid self-nanoemulsifying drug delivery system of lopinavir using design of experiment.Drug Deliv. 2016 Oct;23(8):3027-3042. doi: 10.3109/10717544.2016.1141260. Epub 2016 Feb 16. Drug Deliv. 2016. PMID: 26882014
-
Freeze-Dried Lopinavir-Loaded Nanostructured Lipid Carriers for Enhanced Cellular Uptake and Bioavailability: Statistical Optimization, in Vitro and in Vivo Evaluations.Pharmaceutics. 2019 Feb 25;11(2):97. doi: 10.3390/pharmaceutics11020097. Pharmaceutics. 2019. PMID: 30823545 Free PMC article.
-
Formulation and Evaluation of Linum usitatissimum Mucilage-Based Nanoparticles for Effective Delivery of Ezetimibe.Int J Nanomedicine. 2021 Jul 5;16:4579-4596. doi: 10.2147/IJN.S308790. eCollection 2021. Int J Nanomedicine. 2021. PMID: 34267514 Free PMC article.
-
Formulation, Characterization, and Evaluation of Eudragit-Coated Saxagliptin Nanoparticles Using 3 Factorial Design Modules.Molecules. 2022 Nov 3;27(21):7510. doi: 10.3390/molecules27217510. Molecules. 2022. PMID: 36364338 Free PMC article.
Cited by
-
Superior possibilities and upcoming horizons for nanoscience in COVID-19: noteworthy approach for effective diagnostics and management of SARS-CoV-2 outbreak.Chem Zvesti. 2023 Apr 4:1-24. doi: 10.1007/s11696-023-02795-3. Online ahead of print. Chem Zvesti. 2023. PMID: 37362791 Free PMC article. Review.
-
Lopinavir-menthol co-crystals for enhanced dissolution rate and intestinal absorption.J Drug Deliv Sci Technol. 2022 Aug;74:103587. doi: 10.1016/j.jddst.2022.103587. Epub 2022 Jul 11. J Drug Deliv Sci Technol. 2022. PMID: 35845293 Free PMC article.
References
-
- Abass H., Kamel R., Abdelbary A. Metronidazole bioadhesive vaginal suppositories: formulation, in vitro and in vivo evaluation. Int. J. Pharm. Pharmaceut. Sci. 2012;4:344–355.
-
- Abou-El-Naga I.F., El Kerdany E.D., Mady R.F., Shalaby T.I., Zaytoun E.M. The effect of lopinavir/ritonavir and lopinavir/ritonavir loaded PLGA nanoparticles on experimental toxoplasmosis. Parasitol. Int. 2017;66(6):735–747. - PubMed
-
- Akin-Ajani O.D., Odeku O.A., Babalola Y. Formulation of paediatric paracetamol suppositories using shea butter and dika fat as suppository bases. Trop. J. Nat. Prod. Res. February 2019;3(2):31–36.
-
- Cao J., Bhoyro A.Y. Structural characterization of wool by thermal mechanical analysis of yarns. Tex. Res. J. 2001;71:63–66.
-
- Das Neves J., Amiji M., Bahia M.F. Assessing the physical–chemical properties and stability of dapivirine-loaded polymeric nanoparticles. Int. J. Pharm. 2013;456:307–314. - PubMed
LinkOut - more resources
Full Text Sources

