Gene-activated dermal equivalents to accelerate healing of diabetic chronic wounds by regulating inflammation and promoting angiogenesis
- PMID: 32420517
- PMCID: PMC7217806
- DOI: 10.1016/j.bioactmat.2020.04.018
Gene-activated dermal equivalents to accelerate healing of diabetic chronic wounds by regulating inflammation and promoting angiogenesis
Abstract
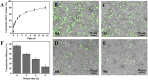
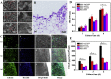
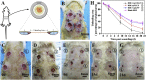
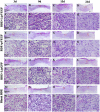
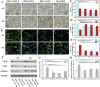
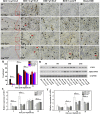
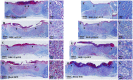
Diabetic chronic wound, characterized by prolonged inflammation and impaired angiogenesis, has become one of the most serious challenges in clinic and pose a significant healthcare burden worldwide. Although a great variety of wound dressings have been developed, few of encouraged achievements were obtained so far. In this study, the gene-activated strategy was applied to enhance sustained expression of vascular endothelial growth factor (VEGF) and achieve better healing outcomes by regulating inflammation and promoting angiogenesis. The gene-activated bilayer dermal equivalents (Ga-BDEs), which has good biocompatibility, were fabricated by loading the nano-sized complexes of Lipofectamine 2000/plasmid DNA-encoding VEGF into a collagen-chitosan scaffold/silicone membrane bilayer dermal equivalent. The DNA complexes were released in a sustained manner and showed the effective transfection capacities to up-regulate the expression of VEGF in vitro. To overcome cutaneous contraction of rodents and mimic the wound healing mechanisms of the human, a reformative rat model of full-thickness diabetic chronic wound was adopted. Under the treatment of Ga-BDEs, speeding wound healing was observed, which is accompanied by the accelerated infiltration and phenotype shift of macrophages and enhanced angiogenesis in early and late healing phases, respectively. These proved that Ga-BDEs possess the functions of immunomodulation and pro-angiogenesis simultaneously. Subsequently, the better regeneration outcomes, including deposition of oriented collagen and fast reepithelialization, were achieved. All these results indicated that, being different from traditional pro-angiogenic concept, the up-regulated expression of VEGF by Ga-BDEs in a sustained manner shows versatile potentials for promoting the healing of diabetic chronic wounds.
Keywords: Angiogenesis; Diabetic chronic wounds; Gene-activated dermal equivalent; Inflammation; Vascular endothelial growth factor.
© 2020 Production and hosting by Elsevier B.V. on behalf of KeAi Communications Co., Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Ogurtsova K., Da R.F.J., Huang Y., Linnenkamp U., Guariguata L., Cho N.H., Cavan D., Shaw J.E., Makaroff L.E. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017;128:40–50. - PubMed
-
- Baltzis D., Eleftheriadou I., Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv. Ther. 2014;31:817–836. - PubMed
-
- Boulton A.J. The pathway to foot ulceration in diabetes. Med Clin North Am. 2013;97:775–790. - PubMed
-
- Mulder G.D., Patt L.M., Sanders L., Rosenstock J., Altman M.I., Hanley M.E., Duncan G.W. Enhanced healing of ulcers in patients with diabetes by topical treatment with glycyl-l-histidyl-l-lysine copper. Wound Repair Regen. 1994;2:259–269. - PubMed
-
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. - PubMed
LinkOut - more resources
Full Text Sources

