HIF1α-dependent mitophagy facilitates cardiomyoblast differentiation
- PMID: 32420530
- PMCID: PMC7212530
- DOI: 10.15698/cst2020.05.220
HIF1α-dependent mitophagy facilitates cardiomyoblast differentiation
Abstract
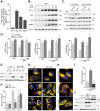
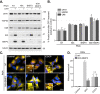
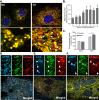
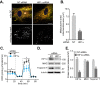
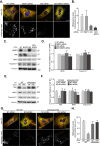
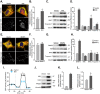
Mitophagy is thought to play a key role in eliminating damaged mitochondria, with diseases such as cancer and neurodegeneration exhibiting defects in this process. Mitophagy is also involved in cell differentiation and maturation, potentially through modulating mitochondrial metabolic reprogramming. Here we examined mitophagy that is induced upon iron chelation and found that the transcriptional activity of HIF1α, in part through upregulation of BNIP3 and NIX, is an essential mediator of this pathway in SH-SY5Y cells. In contrast, HIF1α is dispensable for mitophagy occurring upon mitochondrial depolarisation. To examine the role of this pathway in a metabolic reprogramming and differentiation context, we utilised the H9c2 cell line model of cardiomyocyte maturation. During differentiation of these cardiomyoblasts, mitophagy increased and required HIF1α-dependent upregulation of NIX. Though HIF1α was essential for expression of key cardiomyocyte markers, mitophagy was not directly required. However, enhancing mitophagy through NIX overexpression, accelerated marker gene expression. Taken together, our findings provide a molecular link between mitophagy signalling and cardiomyocyte differentiation and suggest that although mitophagy may not be essential per se, it plays a critical role in maintaining mitochondrial integrity during this energy demanding process.
Keywords: BNIP3; HIF1α; NIX; cardiomyocyte; differentiation; iron chelation; mitophagy.
Copyright: © 2020 Zhao et al.
Conflict of interest statement
Conflict of interest: The authors declare they have no conflict of interest.
Figures


Comment in
-
HIF1α or mitophagy: which drives cardiomyocyte differentiation?Cell Stress. 2020 May 11;4(5):95-98. doi: 10.15698/cst2020.05.219. Cell Stress. 2020. PMID: 32421042 Free PMC article. No abstract available.
References
Grants and funding
LinkOut - more resources
Full Text Sources
