A γ-Lactam Siderophore Antibiotic Effective against Multidrug-Resistant Gram-Negative Bacilli
- PMID: 32420736
- PMCID: PMC7590959
- DOI: 10.1021/acs.jmedchem.0c00255
A γ-Lactam Siderophore Antibiotic Effective against Multidrug-Resistant Gram-Negative Bacilli
Abstract
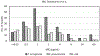
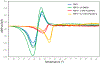
Treatment of multidrug-resistant Gram-negative bacterial pathogens represents a critical clinical need. Here, we report a novel γ-lactam pyrazolidinone that targets penicillin-binding proteins (PBPs) and incorporates a siderophore moiety to facilitate uptake into the periplasm. The MIC values of γ-lactam YU253434, 1, are reported along with the finding that 1 is resistant to hydrolysis by all four classes of β-lactamases. The druglike characteristics and mouse PK data are described along with the X-ray crystal structure of 1 binding to its target PBP3.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





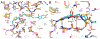

Similar articles
-
A γ-lactam siderophore antibiotic effective against multidrug-resistant Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter spp.Eur J Med Chem. 2021 Aug 5;220:113436. doi: 10.1016/j.ejmech.2021.113436. Epub 2021 Apr 8. Eur J Med Chem. 2021. PMID: 33933754 Free PMC article.
-
Structural Characterization of Diazabicyclooctane β-Lactam "Enhancers" in Complex with Penicillin-Binding Proteins PBP2 and PBP3 of Pseudomonas aeruginosa.mBio. 2021 Feb 16;12(1):e03058-20. doi: 10.1128/mBio.03058-20. mBio. 2021. PMID: 33593978 Free PMC article.
-
Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli.Drugs. 2019 Feb;79(3):271-289. doi: 10.1007/s40265-019-1055-2. Drugs. 2019. PMID: 30712199 Review.
-
Structural Insights into Ceftobiprole Inhibition of Pseudomonas aeruginosa Penicillin-Binding Protein 3.Antimicrob Agents Chemother. 2020 Apr 21;64(5):e00106-20. doi: 10.1128/AAC.00106-20. Print 2020 Apr 21. Antimicrob Agents Chemother. 2020. PMID: 32152075 Free PMC article.
-
Cefiderocol: A Novel Siderophore Cephalosporin against Multidrug-Resistant Gram-Negative Pathogens.Pharmacotherapy. 2020 Dec;40(12):1228-1247. doi: 10.1002/phar.2476. Epub 2020 Nov 19. Pharmacotherapy. 2020. PMID: 33068441 Review.
Cited by
-
A γ-lactam siderophore antibiotic effective against multidrug-resistant Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter spp.Eur J Med Chem. 2021 Aug 5;220:113436. doi: 10.1016/j.ejmech.2021.113436. Epub 2021 Apr 8. Eur J Med Chem. 2021. PMID: 33933754 Free PMC article.
-
Penicillin-binding protein (PBP) inhibitor development: A 10-year chemical perspective.Exp Biol Med (Maywood). 2023 Oct;248(19):1657-1670. doi: 10.1177/15353702231208407. Epub 2023 Nov 29. Exp Biol Med (Maywood). 2023. PMID: 38030964 Free PMC article. Review.
-
Novel analogues of a nonnucleoside SARS-CoV-2 RdRp inhibitor as potential antivirotics.Beilstein J Org Chem. 2024 May 6;20:1029-1036. doi: 10.3762/bjoc.20.91. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 38746653 Free PMC article.
-
Structural Characterization of Diazabicyclooctane β-Lactam "Enhancers" in Complex with Penicillin-Binding Proteins PBP2 and PBP3 of Pseudomonas aeruginosa.mBio. 2021 Feb 16;12(1):e03058-20. doi: 10.1128/mBio.03058-20. mBio. 2021. PMID: 33593978 Free PMC article.
-
Advances in Understanding of the Copper Homeostasis in Pseudomonas aeruginosa.Int J Mol Sci. 2021 Feb 19;22(4):2050. doi: 10.3390/ijms22042050. Int J Mol Sci. 2021. PMID: 33669570 Free PMC article. Review.
References
-
- Ctrol and Prevention (2019) Antibiotic Resistance Threats in the United States, 2019 AR Threats Report https://www.cdc.gov/drugresistance/biggest-threats.html (accessed Mar 11, 2020)
- Cassini A; Högberg LD; Plachouras D; Quattrocchi A; Hoxha A; Simonsen GS; Colomb-Cotinat M; Kretzschmar ME; Devleesschauwer B; Cecchini M; Ouakrim DA; Oliveira TC; Struelens MJ; Suetens C; Monnet DL; Burden of AMR Collaborative Group. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infectious Disease 2019, 19, 56–66. - PMC - PubMed
-
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance, 2014. https://www.who.int/antimicrobial-resistance/publications/surveillancere... (accessed Mar 11, 2020).
-
- Spellberg B; Guidos R; Gilbert D; Bradley J; Boucher HW; Scheld WM; Bartlett JG; Edwards J Jr.; The Infectious Diseases Society of America. The epidemic of antibiotic-resistant infections: a call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis 2008, 46, 155–164. - PubMed
-
- Boucher HW; Talbot GH; Bradley JS; Edwards JE; Gilbert D; Rice LB; Scheld M; Spellberg B; Bartlett J Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis 2009, 48, 1–12. - PubMed
-
- World Health Organization. Global Priority List of Antibiotic-resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics, 2017. https://www.who.int/medicines/publications/global-priority-list-antibiot... (accessed Mar 11, 2020).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Medical

