Structure-Guided Optimization of Inhibitors of Acetyltransferase Eis from Mycobacterium tuberculosis
- PMID: 32421305
- PMCID: PMC7385556
- DOI: 10.1021/acschembio.0c00184
Structure-Guided Optimization of Inhibitors of Acetyltransferase Eis from Mycobacterium tuberculosis
Abstract
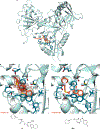
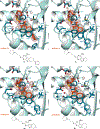
The enhanced intracellular survival (Eis) protein of Mycobacterium tuberculosis (Mtb) is a versatile acetyltransferase that multiacetylates aminoglycoside antibiotics abolishing their binding to the bacterial ribosome. When overexpressed as a result of promoter mutations, Eis causes drug resistance. In an attempt to overcome the Eis-mediated kanamycin resistance of Mtb, we designed and optimized structurally unique thieno[2,3-d]pyrimidine Eis inhibitors toward effective kanamycin adjuvant combination therapy. We obtained 12 crystal structures of enzyme-inhibitor complexes, which guided our rational structure-based design of 72 thieno[2,3-d]pyrimidine analogues divided into three families. We evaluated the potency of these inhibitors in vitro as well as their ability to restore the activity of kanamycin in a resistant strain of Mtb, in which Eis was upregulated. Furthermore, we evaluated the metabolic stability of 11 compounds in vitro. This study showcases how structural information can guide Eis inhibitor design.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


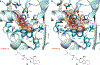

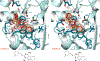




References
-
- Rice LB (2008) Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis 197, 1079–1081. - PubMed
-
- Global Tuberculosis Report 2019, World Health Organization, Geneva, Switzerland, 2019, licence CCBY-NC-SA3.01GO.
-
- Campbell PJ, Morlock GP, Sikes RD, Dalton TL, Metchock B, Starks AM, Hooks DP, Cowan LS, Plikaytis BB, and Posey JE (2011) Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrob. Agents Chemother 55, 2032–2041. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

