Implementation of Minimally Invasive Esophagectomy From a Randomized Controlled Trial Setting to National Practice
- PMID: 32421440
- PMCID: PMC7325364
- DOI: 10.1200/JCO.19.02483
Implementation of Minimally Invasive Esophagectomy From a Randomized Controlled Trial Setting to National Practice
Abstract
Purpose: The aim of this study was to examine the external validity of the randomized TIME trial, when minimally invasive esophagectomy (MIE) was implemented nationally in the Netherlands, using data from the Dutch Upper GI Cancer Audit (DUCA) for transthoracic esophagectomy.
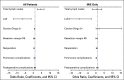
Methods: Original patient data from the TIME trial were extracted along with data from the DUCA dataset (2011-2017). Multivariate analysis, with adjustment for patient factors, tumor factors, and year of surgery, was performed for the effect of MIE versus open esophagectomy on clinical outcomes.
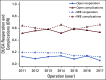
Results: One hundred fifteen patients from the TIME trial (59 MIE v 56 open) and 4,605 patients from the DUCA dataset (2,652 MIE v 1,953 open) were included. In the TIME trial, univariate analysis showed that MIE reduced pulmonary complications and length of hospital stay. On the contrary, in the DUCA dataset, MIE was associated with increased total and pulmonary complications and reoperations; however, benefits included increased proportion of R0 margin and lymph nodes harvested, and reduced 30-day mortality. Multivariate analysis from the TIME trial showed that MIE reduced pulmonary complications (odds ratio [OR], 0.19; 95% CI, 0.06 to 0.61). In the DUCA dataset, MIE was associated with increased total complications (OR, 1.36; 95% CI, 1.19 to 1.57), pulmonary complications (OR, 1.50; 95% CI, 1.29 to 1.74), reoperations (OR, 1.74; 95% CI, 1.42 to 2.14), and length of hospital stay. Multivariate analysis of the combined and MIE datasets showed that inclusion in the TIME trial was associated with a reduction in reoperations, Clavien-Dindo grade > 1 complications, and length of hospital stay.
Conclusion: When adopted nationally outside the TIME trial, MIE was associated with an increase in total and pulmonary complications and reoperation rate. This may reflect nonexpert surgeons outside of high-volume centers performing this minimally invasive technique in a nonstandardized fashion outside of a controlled environment.
Figures



Comment in
-
Innovations in Surgical Technique and Translation to Broad Clinical Practice.J Clin Oncol. 2020 Jul 1;38(19):2119-2121. doi: 10.1200/JCO.20.00885. Epub 2020 May 18. J Clin Oncol. 2020. PMID: 32421441 No abstract available.
-
Reply to B. P. L. Wijnhoven et al and F. Nuytens et al.J Clin Oncol. 2021 Jan 1;39(1):92-93. doi: 10.1200/JCO.20.02354. Epub 2020 Sep 18. J Clin Oncol. 2021. PMID: 32946350 No abstract available.
-
Reply to B. P. L. Wijnhoven et al and F. Nuytens et al.J Clin Oncol. 2021 Jan 1;39(1):93-94. doi: 10.1200/JCO.20.02390. Epub 2020 Sep 18. J Clin Oncol. 2021. PMID: 32946358 No abstract available.
-
Hybrid Minimally Invasive Esophagectomy to the Rescue: A Valid Alternative for Phased Dissemination of TMIE?J Clin Oncol. 2021 Jan 1;39(1):91-92. doi: 10.1200/JCO.20.01964. Epub 2020 Sep 18. J Clin Oncol. 2021. PMID: 32946359 No abstract available.
-
Minimally Invasive Esophagectomy: Time to Reflect on Contemporary Outcomes.J Clin Oncol. 2021 Jan 1;39(1):90-91. doi: 10.1200/JCO.20.01620. Epub 2020 Sep 18. J Clin Oncol. 2021. PMID: 32946360 No abstract available.
References
-
- Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: A systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568. - PMC - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Bouras G, Markar SR, Burns EM, et al. The psychological impact of symptoms related to esophagogastric cancer resection presenting in primary care: A national linked database study. Eur J Surg Oncol. 2017;43:454–460. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

