Regulation and function of V-ATPases in physiology and disease
- PMID: 32422136
- PMCID: PMC7508768
- DOI: 10.1016/j.bbamem.2020.183341
Regulation and function of V-ATPases in physiology and disease
Abstract
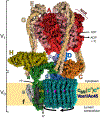
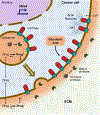
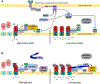
The vacuolar H+-ATPases (V-ATPases) are essential, ATP-dependent proton pumps present in a variety of eukaryotic cellular membranes. Intracellularly, V-ATPase-dependent acidification functions in such processes as membrane traffic, protein degradation, autophagy and the coupled transport of small molecules. V-ATPases at the plasma membrane of certain specialized cells function in such processes as bone resorption, sperm maturation and urinary acidification. V-ATPases also function in disease processes such as pathogen entry and cancer cell invasiveness, while defects in V-ATPase genes are associated with disorders such as osteopetrosis, renal tubular acidosis and neurodegenerative diseases. This review highlights recent advances in our understanding of V-ATPase structure, mechanism, function and regulation, with an emphasis on the signaling pathways controlling V-ATPase assembly in mammalian cells. The role of V-ATPases in cancer and other human pathologies, and the prospects for therapeutic intervention, are also discussed.
Keywords: Acidification; Cancer metastasis; Nutrient sensing; Proton transport; Regulated assembly; Vacuolar ATPase.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
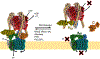
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

