Endogenous opioid peptides in the descending pain modulatory circuit
- PMID: 32422213
- PMCID: PMC7313723
- DOI: 10.1016/j.neuropharm.2020.108131
Endogenous opioid peptides in the descending pain modulatory circuit
Abstract
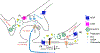
The opioid epidemic has led to a serious examination of the use of opioids for the treatment of pain. Opioid drugs are effective due to the expression of opioid receptors throughout the body. These receptors respond to endogenous opioid peptides that are expressed as polypeptide hormones that are processed by proteolytic cleavage. Endogenous opioids are expressed throughout the peripheral and central nervous system and regulate many different neuronal circuits and functions. One of the key functions of endogenous opioid peptides is to modulate our responses to pain. This review will focus on the descending pain modulatory circuit which consists of the ventrolateral periaqueductal gray (PAG) projections to the rostral ventromedial medulla (RVM). RVM projections modulate incoming nociceptive afferents at the level of the spinal cord. Stimulation within either the PAG or RVM results in analgesia and this circuit has been studied in detail in terms of the actions of exogenous opioids, such as morphine and fentanyl. Further emphasis on understanding the complex regulation of endogenous opioids will help to make rational decisions with regard to the use of opioids for pain. We also include a discussion of the actions of endogenous opioids in the amygdala, an upstream brain structure that has reciprocal connections to the PAG that contribute to the brain's response to pain.
Keywords: Amygdala; Beta-endorphin; Enkephalin; Opioid; Pain; Periaqueductal gray; Rostral ventromedial medulla.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures

References
-
- Ackley MA, Hurley RW, Virnich DE, and Hammond DL (2001). A cellular mechanism for the antinociceptive effect of a kappa opioid receptor agonist. Pain 91, 377–388. - PubMed
-
- Adams JE (1976a). Naloxone reversal of analgesia produced by brain stimulation in the human. Pain 2, 161–166. - PubMed
-
- Adams JE (1976b). Naloxone reversal of analgesia produced by brain stimulation in the human. Pain 2, 161–166. - PubMed
-
- Akil H, Mayer DJ, and Liebeskind JC (1976). Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science 191, 961–962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

