Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study
- PMID: 32422408
- PMCID: PMC7227531
- DOI: 10.1016/j.cmi.2020.05.001
Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study
Abstract
Objectives: Amid the increasing number of pandemic coronavirus disease 2019 (COVID-19) cases, there is a need for a quick and easy method to obtain a non-invasive sample for the detection of this novel coronavirus (severe acute respiratory syndrome coronavirus 2; SARS-CoV-2). We aimed to investigate the potential use of saliva samples as a non-invasive tool for the diagnosis of COVID-19.
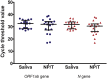
Methods: From 27 March to 4 April 2020, we prospectively collected saliva samples and a standard nasopharyngeal and throat swab in persons seeking care at an acute respiratory infection clinic in a university hospital during the outbreak of COVID-19. Real-time polymerase chain reaction (RT-PCR) was performed, and the results of the two specimens were compared.
Results: Two-hundred pairs of samples were collected. Sixty-nine (34.5%) individuals were male, and the median (interquartile) age was 36 (28-48) years. Using nasopharyngeal and throat swab RT-PCR as the reference standard, the prevalence of COVID-19 diagnosed by nasopharyngeal and throat swab RT-PCR was 9.5%. The sensitivity and specificity of the saliva sample RT-PCR were 84.2% (95% CI 60.4%-96.6%), and 98.9% (95% CI 96.1%-99.9%), respectively. An analysis of the agreement between the two specimens demonstrated 97.5% observed agreement (κ coefficient 0.851, 95% CI 0.723-0.979; p < 0.001).
Conclusions: Saliva might be an alternative specimen for the diagnosis of COVID-19. The collection is non-invasive, and non-aerosol generating. This method could facilitate the diagnosis of the disease, given the simplicity of specimen collection and good diagnostic performance.
Keywords: Coronavirus disease 2019; Nasopharyngeal swab; RT-PCR; Saliva; Severe acute respiratory syndrome coronavirus 2; Throat swab.
© 2020 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures
References
-
- World Health Organization . 2020. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases.https://www.who.int/publications-detail/laboratory-testing-for-2019-nove... Available from:
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous