Communication in the Cancer Microenvironment as a Target for Therapeutic Interventions
- PMID: 32422889
- PMCID: PMC7281160
- DOI: 10.3390/cancers12051232
Communication in the Cancer Microenvironment as a Target for Therapeutic Interventions
Abstract
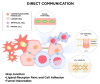
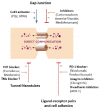
The tumor microenvironment (TME) is a complex system composed of multiple cells, such as non-cancerous fibroblasts, adipocytes, immune and vascular cells, as well as signal molecules and mediators. Tumor cells recruit and reprogram other cells to produce factors that maintain tumor growth. Communication between cancerous and surrounding cells is a two-way process and engages a diverse range of mechanisms that, in consequence, can lead to rapid proliferation, metastasis, and drug resistance, or can serve as a tumors-suppressor, e.g., through tumor-immune cell interaction. Cross-talk within the cancer microenvironment can be direct by cell-to-cell contact via adhesion molecules, electrical coupling, and passage through gap junctions, or indirect through classical paracrine signaling by cytokines, growth factors, and extracellular vesicles. Therapeutic approaches for modulation of cell-cell communication may be a promising strategy to combat tumors. In particular, integrative approaches targeting tumor communication in combination with conventional chemotherapy seem reasonable. Currently, special attention is paid to suppressing the formation of open-ended channels as well as blocking exosome production or ablating their cargos. However, many aspects of cell-to-cell communication have yet to be clarified, and, in particular, more work is needed in regard to mechanisms of bidirectional signal transfer. Finally, it seems that some interactions in TEM can be not only cancer-specific, but also patient-specific, and their recognition would help to predict patient response to therapy.
Keywords: communication in cancer; oncology therapy; therapeutic target; tumor microenvironment.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

