MiR-519d-3p in Trophoblastic Cells: Effects, Targets and Transfer to Allogeneic Immune Cells via Extracellular Vesicles
- PMID: 32422900
- PMCID: PMC7278925
- DOI: 10.3390/ijms21103458
MiR-519d-3p in Trophoblastic Cells: Effects, Targets and Transfer to Allogeneic Immune Cells via Extracellular Vesicles
Abstract
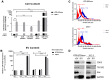
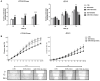
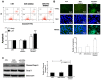
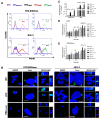
Members of the placenta-specific miRNA cluster C19MC, including miR-519d, are secreted by fetal trophoblast cells within extracellular vesicles (EVs). Trophoblast-derived EVs can be internalized by the autologous trophoblast and surrounding maternal immune cells, resulting in coordination of cellular responses. The study of functions and targets of placental miRNAs in the donor and recipient cells may contribute to the understanding of the immune tolerance essential in pregnancy. Here, we report that miR-519d-3p levels correlate positively with cell proliferation and negatively with migration in trophoblastic cell lines. Inhibition of miR-519d-3p in JEG-3 cells increases caspase-3 activation and apoptosis. PDCD4 and PTEN are targeted by miR-519d-3p in a cell type-specific manner. Transfection of trophoblastic cell lines with miR-519d mimic results in secretion of EVs containing elevated levels of this miRNA (EVmiR-519d). Autologous cells enhance their proliferation and decrease their migration ability when treated with EVmiR-519d. NK92 cells incorporate EV-delivered miR-519d-3p at higher levels than Jurkat T cells. EVmiR-519d increases the proliferation of Jurkat T cells but decreases that of NK92 cells. Altogether, miR-519d-3p regulates pivotal trophoblast cell functions, can be transferred horizontally via EVs to maternal immune cells and exerts functions therein. Vesicular miRNA transfer from fetal trophoblasts to maternal immune cells may contribute to the immune tolerance in pregnancy.
Keywords: C19MC; NK cells; T cells; cell–cell communication; extracellular vesicles; pregnancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
MiR-519d-3p from Placenta-Derived Exosomes Induce Immune Intolerance Regulating Immune Cells, Contributing to the Pathogenesis of Preeclampsia.Immunol Invest. 2025 May;54(4):522-543. doi: 10.1080/08820139.2025.2450234. Epub 2025 Jan 17. Immunol Invest. 2025. PMID: 39819170
-
MicroRNA-141 is upregulated in preeclamptic placentae and regulates trophoblast invasion and intercellular communication.Transl Res. 2016 Jun;172:61-72. doi: 10.1016/j.trsl.2016.02.012. Epub 2016 Mar 4. Transl Res. 2016. PMID: 27012474
-
Dissimilar microRNA-21 functions and targets in trophoblastic cell lines of different origin.Int J Biochem Cell Biol. 2015 Nov;68:187-96. doi: 10.1016/j.biocel.2015.08.018. Epub 2015 Aug 29. Int J Biochem Cell Biol. 2015. PMID: 26320576
-
The function of miR-519d in cell migration, invasion, and proliferation suggests a role in early placentation.Placenta. 2016 Dec;48:34-37. doi: 10.1016/j.placenta.2016.10.004. Epub 2016 Oct 10. Placenta. 2016. PMID: 27871470 Free PMC article. Review.
-
Immunomodulatory properties of extracellular vesicles in the dialogue between placental and immune cells.Am J Reprod Immunol. 2021 Feb;85(2):e13383. doi: 10.1111/aji.13383. Epub 2020 Dec 27. Am J Reprod Immunol. 2021. PMID: 33251688 Review.
Cited by
-
Human Cytomegalovirus Modifies Placental Small Extracellular Vesicle Composition to Enhance Infection of Fetal Neural Cells In Vitro.Viruses. 2022 Sep 13;14(9):2030. doi: 10.3390/v14092030. Viruses. 2022. PMID: 36146834 Free PMC article.
-
Epithelial Membrane Protein 2 Suppresses Non-Small Cell Lung Cancer Cell Growth by Inhibition of MAPK Pathway.Int J Mol Sci. 2021 Mar 14;22(6):2944. doi: 10.3390/ijms22062944. Int J Mol Sci. 2021. PMID: 33799364 Free PMC article.
-
Placenta Extracellular Vesicles: Messengers Connecting Maternal and Fetal Systems.Biomolecules. 2024 Aug 13;14(8):995. doi: 10.3390/biom14080995. Biomolecules. 2024. PMID: 39199382 Free PMC article. Review.
-
Synergies of Extracellular Vesicles and Microchimerism in Promoting Immunotolerance During Pregnancy.Front Immunol. 2022 Jul 1;13:837281. doi: 10.3389/fimmu.2022.837281. eCollection 2022. Front Immunol. 2022. PMID: 35844513 Free PMC article. Review.
-
Identification and validation of aging-related genes in COPD based on bioinformatics analysis.Aging (Albany NY). 2022 May 24;14(10):4336-4356. doi: 10.18632/aging.204064. Epub 2022 May 24. Aging (Albany NY). 2022. PMID: 35609226 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- grant Mo2017/2 and Mo2017/3 to DMMP and Ma1550/12-1 to URM and RFR/Deutsche Forschungsgemeinschaft
- junior project J54, #973652/Interdisziplinäres Zentrum für Klinische Forschung, Universitätsklinikum Jena
- WC, SMP and RNGS have received Ph.D. grants/Deutscher Akademischer Austauschdienst
- CVU: 446429 and register no. 293925/Consejo Nacional de Ciencia y Tecnología
- 91655120/Deutscher Akademischer Austauschdienst
LinkOut - more resources
Full Text Sources
Research Materials

