Acute Effects of Butyrate on Induced Hyperpermeability and Tight Junction Protein Expression in Human Colonic Tissues
- PMID: 32422994
- PMCID: PMC7277647
- DOI: 10.3390/biom10050766
Acute Effects of Butyrate on Induced Hyperpermeability and Tight Junction Protein Expression in Human Colonic Tissues
Abstract
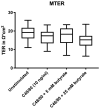
Intact intestinal barrier function is essential for maintaining intestinal homeostasis. A dysfunctional intestinal barrier can lead to local and systemic inflammation through translocation of luminal antigens and has been associated with a range of health disorders. Butyrate, a short-chain fatty acid derived from microbial fermentation of dietary fibers in the colon, has been described as an intestinal barrier-strengthening agent, although mainly by using in vitro and animal models. This study aimed to investigate butyrate's ability to prevent intestinal hyperpermeability, induced by the mast cell degranulator Compound 48/80 (C48/80), in human colonic tissues. Colonic biopsies were collected from 16 healthy subjects and intestinal permeability was assessed by Ussing chamber experiments. Furthermore, the expression levels of tight junction-related proteins were determined by quantitative reverse transcription polymerase chain reaction (qRT-PCR). Pre-treatment with 5 mM butyrate or 25 mM butyrate did not protect the colonic tissue against induced paracellular or transcellular hyperpermeability, measured by FITC-dextran and horseradish peroxidase passage, respectively. Biopsies treated with 25 mM butyrate prior to stimulation with C48/80 showed a reduced expression of claudin 1. In conclusion, this translational ex vivo study did not demonstrate an acute protective effect of butyrate against a chemical insult to the intestinal barrier in healthy humans.
Keywords: Ussing chamber; butyrate; intestinal barrier function; intestinal permeability; tight junctions.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
-
- Guo S., Al-Sadi R., Said H.M., Ma T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2012;182:375–387. doi: 10.1016/j.ajpath.2012.10.014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

