Evanescent-Wave Fiber Optic Sensing of the Anionic Dye Uranine Based on Ion Association Extraction
- PMID: 32423008
- PMCID: PMC7287843
- DOI: 10.3390/s20102796
Evanescent-Wave Fiber Optic Sensing of the Anionic Dye Uranine Based on Ion Association Extraction
Abstract
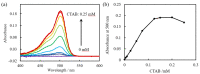
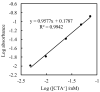
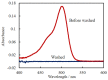
Herein, we propose an evanescent-wave fiber optic sensing technique for the anionic dye uranine based on ion association extraction. The sensor was prepared by removing a section of the cladding from a multimode fiber and hydrophobization of the exposed core surface. Uranine was extracted in association along with hexadecyltrimethylammonium (CTA) ion onto the fiber surface and detected via absorption of the evanescent wave generated on the surface of the exposed fiber core. The effect of CTA+ concentration added for ion association was investigated, revealing that the absorbance of uranine increased with increasing CTA+ concentration. A change in the sensor response as a function of the added uranine concentration was clearly observed. The extraction data were analyzed using a distribution equilibrium model and a Freundlich isotherm. The uranine concentration in the evanescent field of the fiber optic was up to 54 times higher than that in the bulk solution, and the limit of detection (3σ) for uranine was found to be 1.3 nM.
Keywords: evanescent-wave fiber optic sensor; ion association; uranine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Small-volume fiber-optic evanescent-wave absorption sensor for nitrite determination.Anal Bioanal Chem. 2010 Jan;396(2):943-8. doi: 10.1007/s00216-009-3246-2. Epub 2009 Nov 10. Anal Bioanal Chem. 2010. PMID: 19902188
-
Determination of bacterial activity by use of an evanescent-wave fiber-optic sensor.Appl Opt. 2002 Dec 1;41(34):7334-8. doi: 10.1364/ao.41.007334. Appl Opt. 2002. PMID: 12477126
-
Optical sensor for fluoride determination in tea sample based on evanescent-wave interaction and fiber-optic integration.Talanta. 2017 Nov 1;174:372-379. doi: 10.1016/j.talanta.2017.06.024. Epub 2017 Jun 13. Talanta. 2017. PMID: 28738594
-
Evanescent wave biosensors. Real-time analysis of biomolecular interactions.Mol Biotechnol. 1995 Feb;3(1):47-54. doi: 10.1007/BF02821334. Mol Biotechnol. 1995. PMID: 7606504 Review.
-
Recent Progress in Functional-Nucleic-Acid-Based Fluorescent Fiber-Optic Evanescent Wave Biosensors.Biosensors (Basel). 2023 Mar 27;13(4):425. doi: 10.3390/bios13040425. Biosensors (Basel). 2023. PMID: 37185500 Free PMC article. Review.
Cited by
-
A Portable 'Plug-and-Play' Fibre Optic Sensor for In-Situ Measurements of pH Values for Microfluidic Applications.Micromachines (Basel). 2022 Jul 30;13(8):1224. doi: 10.3390/mi13081224. Micromachines (Basel). 2022. PMID: 36014146 Free PMC article.
-
Advancements in Optical Fiber Sensors for pH Measurement: Technologies and Applications.Sensors (Basel). 2025 Jul 9;25(14):4275. doi: 10.3390/s25144275. Sensors (Basel). 2025. PMID: 40732404 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

