COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males?
- PMID: 32423094
- PMCID: PMC7278991
- DOI: 10.3390/ijms21103474
COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males?
Abstract
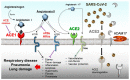
In December 2019, a novel severe acute respiratory syndrome (SARS) from a new coronavirus (SARS-CoV-2) was recognized in the city of Wuhan, China. Rapidly, it became an epidemic in China and has now spread throughout the world reaching pandemic proportions. High mortality rates characterize SARS-CoV-2 disease (COVID-19), which mainly affects the elderly, causing unrestrained cytokines-storm and subsequent pulmonary shutdown, also suspected micro thromboembolism events. At the present time, no specific and dedicated treatments, nor approved vaccines, are available, though very promising data come from the use of anti-inflammatory, anti-malaria, and anti-coagulant drugs. In addition, it seems that males are more susceptible to SARS-CoV-2 than females, with males 65% more likely to die from the infection than females. Data from the World Health Organization (WHO) and Chinese scientists show that of all cases about 1.7% of women who contract the virus will die compared with 2.8% of men, and data from Hong Kong hospitals state that 32% of male and 15% of female COVID-19 patients required intensive care or died. On the other hand, the long-term fallout of coronavirus may be worse for women than for men due to social and psychosocial reasons. Regardless of sex- or gender-biased data obtained from WHO and those gathered from sometimes controversial scientific journals, some central points should be considered. Firstly, SARS-CoV-2 has a strong interaction with the human ACE2 receptor, which plays an essential role in cell entry together with transmembrane serine protease 2 (TMPRSS2); it is interesting to note that the ACE2 gene lays on the X-chromosome, thus allowing females to be potentially heterozygous and differently assorted compared to men who are definitely hemizygous. Secondly, the higher ACE2 expression rate in females, though controversial, might ascribe them the worst prognosis, in contrast with worldwide epidemiological data. Finally, several genes involved in inflammation are located on the X-chromosome, which also contains high number of immune-related genes responsible for innate and adaptive immune responses to infection. Other genes, out from the RAS-pathway, might directly or indirectly impact on the ACE1/ACE2 balance by influencing its main actors (e.g., ABO locus, SRY, SOX3, ADAM17). Unexpectedly, the higher levels of ACE2 or ACE1/ACE2 rebalancing might improve the outcome of COVID-19 in both sexes by reducing inflammation, thrombosis, and death. Moreover, X-heterozygous females might also activate a mosaic advantage and show more pronounced sex-related differences resulting in a sex dimorphism, further favoring them in counteracting the progression of the SARS-CoV-2 infection.
Keywords: ACE1; ACE2; COVID-19; RAS-pathway; SARS-CoV-2; TMPRSS2; inflammation; lung shut-down; prognostic molecular/genetic markers; sex/gender-gap; thrombosis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Menachery V.D., Yount B.L., Jr., Debbink K., Agnihothram S., Gralinski L.E., Plante J.A., Graham R.L., Scobey T., Ge X.Y., Donaldson E.F., et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 2015;21:1508–1513. doi: 10.1038/nm.3985. - DOI - PMC - PubMed
-
- Alagaili A.N., Briese T., Mishra N., Kapoor V., Sameroff S.C., Burbelo P.D., de Wit E., Munster V.J., Hensley L.E., Zalmout I.S., et al. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio. 2014;5:e00884-14. doi: 10.1128/mBio.00884-14. - DOI - PMC - PubMed
-
- Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

