CX3CR1-Targeted PLGA Nanoparticles Reduce Microglia Activation and Pain Behavior in Rats with Spinal Nerve Ligation
- PMID: 32423102
- PMCID: PMC7279022
- DOI: 10.3390/ijms21103469
CX3CR1-Targeted PLGA Nanoparticles Reduce Microglia Activation and Pain Behavior in Rats with Spinal Nerve Ligation
Abstract
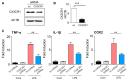
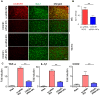
Activation of CX3CR1 in microglia plays an important role in the development of neuropathic pain. Here, we investigated whether neuropathic pain could be attenuated in spinal nerve ligation (SNL)-induced rats by reducing microglial activation through the use of poly(D,L-lactic-co-glycolic acid) (PLGA)-encapsulated CX3CR1 small-interfering RNA (siRNA) nanoparticles. After confirming the efficacy and specificity of CX3CR1 siRNA, as evidenced by its anti-inflammatory effects in lipopolysaccharide-stimulated BV2 cells in vitro, PLGA-encapsulated CX3CR1 siRNA nanoparticles were synthesized by sonication using the conventional double emulsion (W/O/W) method and administered intrathecally into SNL rats. CX3CR1 siRNA-treated rats exhibited significant reductions in the activation of microglia in the spinal dorsal horn and a downregulation of proinflammatory mediators, as well as a significant attenuation of mechanical allodynia. These data indicate that the PLGA-encapsulated CX3CR1 siRNA nanoparticles effectively reduce neuropathic pain in SNL-induced rats by reducing microglial activity and the expression of proinflammatory mediators. Therefore, we believe that PLGA-encapsulated CX3CR1 siRNA nanoparticles represent a valuable new treatment option for neuropathic pain.
Keywords: CX3CR1; Poly(D,L-lactic-co-glycolic acid) (PLGA) nanoparticles; microglia; neuropathic pain; siRNA; spinal nerve ligation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Foxp3 plasmid-encapsulated PLGA nanoparticles attenuate pain behavior in rats with spinal nerve ligation.Nanomedicine. 2019 Jun;18:90-100. doi: 10.1016/j.nano.2019.02.023. Epub 2019 Mar 8. Nanomedicine. 2019. PMID: 30858084
-
IKBKB siRNA-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles Diminish Neuropathic Pain by Inhibiting Microglial Activation.Int J Mol Sci. 2021 May 26;22(11):5657. doi: 10.3390/ijms22115657. Int J Mol Sci. 2021. PMID: 34073390 Free PMC article.
-
p38 siRNA-encapsulated PLGA nanoparticles alleviate neuropathic pain behavior in rats by inhibiting microglia activation.Nanomedicine (Lond). 2018 Jul;13(13):1607-1621. doi: 10.2217/nnm-2018-0054. Nanomedicine (Lond). 2018. PMID: 30028250
-
p38 MAPK, microglial signaling, and neuropathic pain.Mol Pain. 2007 Nov 1;3:33. doi: 10.1186/1744-8069-3-33. Mol Pain. 2007. PMID: 17974036 Free PMC article. Review.
-
Spinal interleukin-10 therapy to treat peripheral neuropathic pain.Neuromodulation. 2012 Nov-Dec;15(6):520-6; discussion 526. doi: 10.1111/j.1525-1403.2012.00462.x. Epub 2012 Jun 1. Neuromodulation. 2012. PMID: 22672183 Free PMC article. Review.
Cited by
-
Targeting Members of the Chemokine Family as a Novel Approach to Treating Neuropathic Pain.Molecules. 2023 Jul 30;28(15):5766. doi: 10.3390/molecules28155766. Molecules. 2023. PMID: 37570736 Free PMC article. Review.
-
A Trojan horse biomimetic delivery system using mesenchymal stem cells for HIF-1α siRNA-loaded nanoparticles on retinal pigment epithelial cells under hypoxia environment.Int J Ophthalmol. 2022 Nov 18;15(11):1743-1751. doi: 10.18240/ijo.2022.11.03. eCollection 2022. Int J Ophthalmol. 2022. PMID: 36404976 Free PMC article.
-
PLGA nanoparticle-mediated anti-inflammatory gene delivery for the treatment of neuropathic pain.Nanomedicine (Lond). 2025 May;20(9):943-954. doi: 10.1080/17435889.2025.2487410. Epub 2025 Apr 5. Nanomedicine (Lond). 2025. PMID: 40186589
-
Microglia-Mediated Neuroinflammation Through Phosphatidylinositol 3-Kinase Signaling Causes Cognitive Dysfunction.Int J Mol Sci. 2025 Jul 25;26(15):7212. doi: 10.3390/ijms26157212. Int J Mol Sci. 2025. PMID: 40806341 Free PMC article. Review.
-
Nanoparticle Targeting with Antibodies in the Central Nervous System.BME Front. 2023 Mar 31;4:0012. doi: 10.34133/bmef.0012. eCollection 2023. BME Front. 2023. PMID: 37849659 Free PMC article. Review.
References
-
- Zhuang Z.-Y., Kawasaki Y., Tan P.-H., Wen Y.-R., Huang J., Ji R.-R. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav. Immun. 2007;21:642–651. doi: 10.1016/j.bbi.2006.11.003. - DOI - PMC - PubMed
-
- Psaroulaki A., Antoniou M., Toumazos P., Mazeris A., Ioannou I., Chochlakis D., Christophi N., Loukaides P., Patsias A., Moschandrea I., et al. Rats as indicators of the presence and dispersal of six zoonotic microbial agents in Cyprus, an island ecosystem: A seroepidemiological study. Trans. R. Soc. Trop. Med. Hyg. 2010;104:733–739. doi: 10.1016/j.trstmh.2010.08.005. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

