Clinical, Radiological, and Laboratory Features of Spinal Cord Involvement in Primary Sjögren's Syndrome
- PMID: 32423153
- PMCID: PMC7290729
- DOI: 10.3390/jcm9051482
Clinical, Radiological, and Laboratory Features of Spinal Cord Involvement in Primary Sjögren's Syndrome
Abstract
Objective: To identify radiological and laboratory hallmarks in patients with primary Sjögren's syndrome (pSS) presenting with spinal cord involvement.
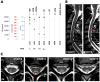
Methods: Clinical and laboratory routine parameters were analyzed in a retrospective multicenter case series of four patients who developed myelitis associated with pSS. Serological and cerebrospinal fluid (CSF) measurements of pSS associated anti-SSA(Ro)-antibodies were initiated, and CSF neurofilament light chain (NFL) levels were assessed. NFL values were compared with results from 15 sex- and age-matched healthy controls. Radiological assessment was performed using multi-sequence spinal cord magnetic resonance imaging.
Results: Three of the four patients initially developed neurological signs suggestive of myelitis and were subsequently diagnosed with pSS. All patients presented a longitudinal spinal T2-hyperintense lesion in the cervical spinal cord, whereas only two patients showed pleocytosis and oligoclonal bands in the CSF. Median (range) CSF-NFL levels were significantly elevated in patients compared to controls (6672 pg/mL (621-50000) vs. 585 pg/mL (357-729), p = 0.009). One patient showed sustained, highly increased NFL levels (50000 pg/mL) in the initial assessment when radiological signs of axonal injury were still absent. Anti-SSA(Ro)-antibodies were found in the serum of three patients, while two patients additionally presented intrathecal anti-SSA(Ro)-antibody production. Elevated CSF-NFL levels and intrathecal synthesis of anti-SSA(Ro)-antibodies were associated with a relapsing and treatment-resistant disease course.
Conclusion: Inflammatory spinal cord lesions associated with pSS are a rare but serious disease leading to severe disability. NFL and anti-SSA(Ro)-antibodies in CSF might serve as prognostic biomarkers and should be routinely assessed in patients with pSS.
Keywords: Antibodies; Cerebrospinal fluid; Myelitis; Neurofilament light chain; Sjögren’s syndrome; Spinal cord.
Conflict of interest statement
M.B. received travel/accommodation/meeting expenses from Shire. J.N., C.B., Ta.S., N.M., T.T., F.F.K. report no conflicts of interest. L.R. received travel reimbursements from Merck Serono and Sanofi Genzyme. M.W. received speaker or consultancy honoraria from Bayer Healthcare, Biogen, Biologix, Celgene, Genilac, Imcyse, IXICO, Medison, Merck-Serono, Novartis, Roche, Sanofi-Genzyme. T.W. receives honoraria for lecturing and travel expenses for attending meetings from Aesku.Diagnostics AbbVie, AMGEN, Biogen, BMS, Celgene, Chugai, CSL Behring, Gilead, GSK, Janssen, Lilly, Medac, MSD, Novartis, Octapharma, Pfizer, Roche Pharma, UCB, Sanofi-Aventis. He holds a patent on the diagnostic application of alpha-fodrin antibodies. His research is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy—EXC 2155 “RESIST”—Project ID 39087428. S.G.M. receives honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology, and by Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche, and Teva. ThS received honoraria for lecturing from Alexion, Bayer Vital, Biogen, Celgene, CSL Behring, Merck, Novartis, Roche, Sanofi Aventis, all outside the submitted work. M.P. received speaker honoraria and travel/accommodation/meeting expenses from Novartis.
Figures

References
-
- Flores-Chávez A., Kostov B., Solans R., Fraile G., Maure B., Feijoo-Massó C., Rascón F.-J., Pérez-Alvarez R., Zamora-Pasadas M., García-Pérez A., et al. Severe, life-threatening phenotype of primary Sjögren’s syndrome: Clinical characterisation and outcomes in 1580 patients (GEAS-SS Registry) Clin. Exp. Rheumatol. 2018;36(Suppl. 112):121–129. - PubMed
-
- Carvajal Alegria G., Guellec D., Mariette X., Gottenberg J.-E., Dernis E., Dubost J.-J., Trouvin A.-P., Hachulla E., Larroche C., Le Guern V., et al. Epidemiology of neurological manifestations in Sjögren’s syndrome: Data from the French ASSESS Cohort. RMD Open. 2016;2:e000179. doi: 10.1136/rmdopen-2015-000179. - DOI - PMC - PubMed
-
- Ramos-Casals M., Solans R., Rosas J., Camps M.T., Gil A., Del Pino-Montes J., Calvo-Alen J., Jiménez-Alonso J., Micó M.-L., Beltrán J., et al. Primary Sjögren syndrome in Spain: Clinical and immunologic expression in 1010 patients. Medicine (Baltimore) 2008;87:210–219. doi: 10.1097/MD.0b013e318181e6af. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

