The Brain's Glymphatic System: Current Controversies
- PMID: 32423764
- PMCID: PMC7331945
- DOI: 10.1016/j.tins.2020.04.003
The Brain's Glymphatic System: Current Controversies
Abstract
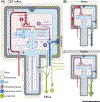
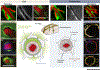
The glymphatic concept along with the discovery of meningeal lymphatic vessels have, in recent years, highlighted that fluid is directionally transported within the central nervous system (CNS). Imaging studies, as well as manipulations of fluid transport, point to a key role of the glymphatic-lymphatic system in clearance of amyloid-β and other proteins. As such, the glymphatic-lymphatic system represents a new target in combating neurodegenerative diseases. Not unexpectedly, introduction of a new plumbing system in the brain has stirred controversies. This opinion article will highlight what we know about the brain's fluid transport systems, where experimental data are lacking, and what is still debated.
Keywords: amyloid-β; aquaporin-4; brain clearance; cerebrospinal fluid; glymphatic system; meningeal lymphatics; perivascular spaces.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors have no conflicts of interest to report.
Figures
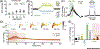
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

