Integrin α3β1 in hair bulge stem cells modulates CCN2 expression and promotes skin tumorigenesis
- PMID: 32423907
- PMCID: PMC7240742
- DOI: 10.26508/lsa.202000645
Integrin α3β1 in hair bulge stem cells modulates CCN2 expression and promotes skin tumorigenesis
Abstract
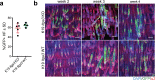
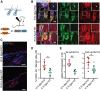
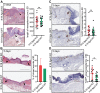
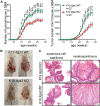
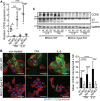
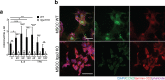
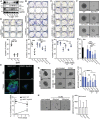
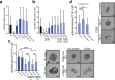
Epidermal-specific deletion of integrin α3β1 almost completely prevents the formation of papillomas during 7,12-Dimethylbenz[ a ]anthracene/12- O -tetradecanoylphorbol-13-acetate (DMBA/TPA) two-stage skin carcinogenesis. This dramatic decrease in tumorigenesis was thought to be due to an egress and premature differentiation of α3β1-depleted hair bulge (HB) stem cells (SCs), previously considered to be the cancer cells-of-origin in the DMBA/TPA model. Using a reporter mouse line with inducible deletion of α3β1 in HBs, we show that HB SCs remain confined to their niche regardless of the presence of α3β1 and are largely absent from skin tumors. However, tumor formation was significantly decreased in mice deficient for α3β1 in HB SCs. RNA sequencing of HB SCs isolated from short-term DMBA/TPA-treated skin showed α3β1-dependent expression of the matricellular protein connective tissue growth factor (CCN2), which was confirmed in vitro, where CCN2 promoted colony formation and 3D growth of transformed keratinocytes. Together, these findings show that HBs contribute to skin tumorigenesis in an α3β1-dependent manner and suggest a role of HB SCs in creating a permissive environment for tumor growth through the modulation of CCN2 secretion.
© 2020 Ramovs et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


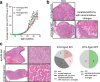
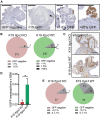
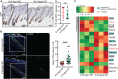
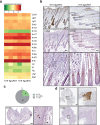
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
