Chlamydia Lipooligosaccharide Has Varied Direct and Indirect Roles in Evading both Innate and Adaptive Host Immune Responses
- PMID: 32423914
- PMCID: PMC7375763
- DOI: 10.1128/IAI.00198-20
Chlamydia Lipooligosaccharide Has Varied Direct and Indirect Roles in Evading both Innate and Adaptive Host Immune Responses
Abstract
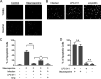
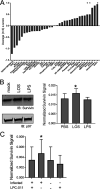
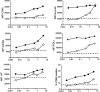
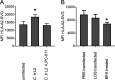
Chlamydia bacteria are obligate intracellular pathogens which can cause a variety of disease in humans and other vertebrate animals. To successfully complete its life cycle, Chlamydia must evade both intracellular innate immune responses and adaptive cytotoxic T cell responses. Here, we report on the role of the chlamydial lipooligosaccharide (LOS) in evading the immune response. Chlamydia infection is known to block the induction of apoptosis. However, when LOS synthesis was inhibited during Chlamydia trachomatis infection, HeLa cells regained susceptibility to apoptosis induction following staurosporine treatment. Additionally, the delivery of purified LOS to the cytosol of cells increased the levels of the antiapoptotic protein survivin. An increase in survivin levels was also detected following C. trachomatis infection, which was reversed by blocking LOS synthesis. Interestingly, while intracellular delivery of lipopolysaccharide (LPS) derived from Escherichia coli was toxic to cells, LOS from C. trachomatis did not induce any appreciable cell death, suggesting that it does not activate pyroptosis. Chlamydial LOS was also a poor stimulator of maturation of bone marrow-derived dendritic cells compared to E. coli LPS. Previous work from our group indicated that LOS synthesis during infection was necessary to alter host cell antigen presentation. However, direct delivery of LOS to cells in the absence of infection did not alter antigenic peptide presentation. Taken together, these data suggest that chlamydial LOS, which is remarkably conserved across the genus Chlamydia, may act both directly and indirectly to allow the pathogen to evade the innate and adaptive immune responses of the host.
Keywords: Chlamydia; antigen processing; apoptosis; dendritic cells.
Copyright © 2020 American Society for Microbiology.
Figures

Similar articles
-
Chlamydia trachomatis Lipopolysaccharide Evades the Canonical and Noncanonical Inflammatory Pathways To Subvert Innate Immunity.mBio. 2019 Apr 23;10(2):e00595-19. doi: 10.1128/mBio.00595-19. mBio. 2019. PMID: 31015326 Free PMC article.
-
A live and inactivated Chlamydia trachomatis mouse pneumonitis strain induces the maturation of dendritic cells that are phenotypically and immunologically distinct.Infect Immun. 2005 Mar;73(3):1568-77. doi: 10.1128/IAI.73.3.1568-1577.2005. Infect Immun. 2005. PMID: 15731055 Free PMC article.
-
Cell death, BAX activation, and HMGB1 release during infection with Chlamydia.Microbes Infect. 2004 Nov;6(13):1145-55. doi: 10.1016/j.micinf.2004.07.004. Microbes Infect. 2004. PMID: 15488733
-
T cell responses to Chlamydia.Pathog Dis. 2021 Mar 31;79(4):ftab014. doi: 10.1093/femspd/ftab014. Pathog Dis. 2021. PMID: 33693620 Free PMC article. Review.
-
Pathogenesis of fallopian tube damage caused by Chlamydia trachomatis infections.Contraception. 2015 Aug;92(2):108-15. doi: 10.1016/j.contraception.2015.01.004. Epub 2015 Jan 13. Contraception. 2015. PMID: 25592078 Review.
Cited by
-
Host cell death during infection with Chlamydia: a double-edged sword.FEMS Microbiol Rev. 2021 Jan 8;45(1):fuaa043. doi: 10.1093/femsre/fuaa043. FEMS Microbiol Rev. 2021. PMID: 32897321 Free PMC article. Review.
-
TargeTron Inactivation of Chlamydia trachomatis gseA Results in a Lipopolysaccharide 3-Deoxy-d-Manno-Oct-2-Ulosonic Acid-Deficient Strain That Is Cytotoxic for Cells.Infect Immun. 2023 Jul 18;91(7):e0009623. doi: 10.1128/iai.00096-23. Epub 2023 May 31. Infect Immun. 2023. PMID: 37255490 Free PMC article.
-
Role of Inflammatory Markers as a Risk Factor for Community-Acquired Pneumonia Management.Medicina (Kaunas). 2025 Jun 11;61(6):1078. doi: 10.3390/medicina61061078. Medicina (Kaunas). 2025. PMID: 40572766 Free PMC article.
-
Insights into innate immune cell evasion by Chlamydia trachomatis.Front Immunol. 2024 Jan 25;15:1289644. doi: 10.3389/fimmu.2024.1289644. eCollection 2024. Front Immunol. 2024. PMID: 38333214 Free PMC article. Review.
-
Genomic Characterization of Bacillus sp. THPS1: A Hot Spring-Derived Species with Functional Features and Biotechnological Potential.Microorganisms. 2024 Dec 2;12(12):2476. doi: 10.3390/microorganisms12122476. Microorganisms. 2024. PMID: 39770679 Free PMC article.
References
-
- Pospischil A, Thoma R, Hilbe M, Grest P, Gebbers JO. 2002. Abortion in woman caused by caprine Chlamydophila abortus (Chlamydia psittaci serovar 1). Swiss Med Wkly 132:64–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

