Mycobacterium abscessus Clearance by Neutrophils Is Independent of Autophagy
- PMID: 32423916
- PMCID: PMC7375761
- DOI: 10.1128/IAI.00024-20
Mycobacterium abscessus Clearance by Neutrophils Is Independent of Autophagy
Abstract
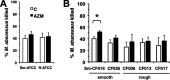
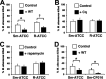
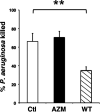
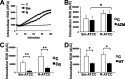
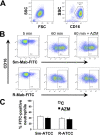
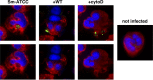
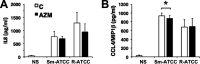
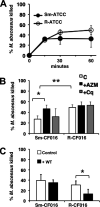
Mycobacterium abscessus, a rapidly growing nontuberculous mycobacterium, is increasingly prevalent in chronic lung disease, including cystic fibrosis, and infections are characterized by neutrophil-dominated environments. However, mechanisms of immune control are poorly understood. Azithromycin, a macrolide antibiotic with immunomodulatory effects, is used to treat M. abscessus infections. Recently, inhibition of macrophage bactericidal autophagy was described for azithromycin, which could be detrimental to the host. Therefore, we explored the role of autophagy in mycobactericidal neutrophils. Azithromycin did not affect M. abscessus-induced neutrophil reactive oxygen species formation, phagocytosis, or cytokine secretion, and neutrophils treated with azithromycin killed M. abscessus equally as well as untreated neutrophils from either healthy or cystic fibrosis subjects. One clinical isolate was killed more effectively in azithromycin-treated neutrophils, suggesting that pathogen-specific factors may interact with an azithromycin-sensitive pathway. Chloroquine and rapamycin, an inhibitor and an activator of autophagy, respectively, also failed to affect mycobactericidal activity, suggesting that autophagy was not involved. However, wortmannin, an inhibitor of intracellular trafficking, inhibited mycobactericidal activity, but as a result of inhibiting phagocytosis. The effects of these autophagy-modifying agents and azithromycin in neutrophils from healthy subjects were similar between the smooth and rough morphotypes of M. abscessus However, in cystic fibrosis neutrophils, wortmannin inhibited killing of a rough clinical isolate and not a smooth isolate, suggesting that unique host-pathogen interactions exist in cystic fibrosis. These studies increase our understanding of M. abscessus virulence and of neutrophil mycobactericidal mechanisms. Insight into the immune control of M. abscessus may provide novel targets of therapy.
Keywords: azithromycin; cell trafficking; chloroquine; cystic fibrosis; innate immunity; phagocytosis; reactive oxygen species; wortmannin.
Copyright © 2020 American Society for Microbiology.
Figures
References
-
- Pierre-Audigier C, Ferroni A, Sermet-Gaudelus I, Le Bourgeois M, Offredo C, Vu-Thien H, Fauroux B, Mariani P, Munck A, Bingen E, Guillemot D, Quesne G, Vincent V, Berche P, Gaillard JL. 2005. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J Clin Microbiol 43:3467–3470. doi: 10.1128/JCM.43.7.3467-3470.2005. - DOI - PMC - PubMed
-
- Qvist T, Gilljam M, Jönsson B, Taylor-Robinson D, Jensen-Fangel S, Wang M, Svahn A, Kötz K, Hansson L, Hollsing A, Hansen CR, Finstad PL, Pressler T, Høiby N, Katzenstein TL, Scandinavian Cystic Fibrosis Study Consortium. 2015. Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. J Cyst Fibros 14:46–52. doi: 10.1016/j.jcf.2014.08.002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

