Development of a long-acting direct-acting antiviral system for hepatitis C virus treatment in swine
- PMID: 32424082
- PMCID: PMC7275718
- DOI: 10.1073/pnas.2004746117
Development of a long-acting direct-acting antiviral system for hepatitis C virus treatment in swine
Abstract
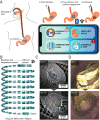
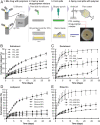
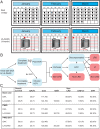
Chronic hepatitis C virus (HCV) infection is a leading cause of cirrhosis worldwide and kills more Americans than 59 other infections, including HIV and tuberculosis, combined. While direct-acting antiviral (DAA) treatments are effective, limited uptake of therapy, particularly in high-risk groups, remains a substantial barrier to eliminating HCV. We developed a long-acting DAA system (LA-DAAS) capable of prolonged dosing and explored its cost-effectiveness. We designed a retrievable coil-shaped LA-DAAS compatible with nasogastric tube administration and the capacity to encapsulate and release gram levels of drugs while resident in the stomach. We formulated DAAs in drug-polymer pills and studied the release kinetics for 1 mo in vitro and in vivo in a swine model. The LA-DAAS was equipped with ethanol and temperature sensors linked via Bluetooth to a phone application to provide patient engagement. We then performed a cost-effectiveness analysis comparing LA-DAAS to DAA alone in various patient groups, including people who inject drugs. Tunable release kinetics of DAAs was enabled for 1 mo with drug-polymer pills in vitro, and the LA-DAAS safely and successfully provided at least month-long release of sofosbuvir in vivo. Temperature and alcohol sensors could interface with external sources for at least 1 mo. The LA-DAAS was cost-effective compared to DAA therapy alone in all groups considered (base case incremental cost-effectiveness ratio $39,800). We believe that the LA-DAA system can provide a cost-effective and patient-centric method for HCV treatment, including in high-risk populations who are currently undertreated.
Conflict of interest statement
Competing interest statement: M.V., J.N.C., J.A.F.S., F.E., C.S., R.K., R.L., and G.T. are co-inventors on multiple patent applications describing gastric resident systems for extended drug release and intragastric sensing. R.L. and G.T. have a financial interest in Lyndra Therapeutics, Inc.; Suono Bio, Inc.; and Celero Systems, Inc., which are biotechnology companies focused on the development of gastrointestinal drug delivery and sensing technologies. Complete details for R.L. can be found at the following link: https://www.dropbox.com/s/yc3xqb5s8s94v7x/Rev%20Langer%20COI.pdf?dl=0. Complete details for G.T. can be found at the following link: https://www.dropbox.com/sh/szi7vnr4a2ajb56/AABs5N5i0q9AfT1IqIJAE-T5a?dl=0. The remaining authors disclose no competing interests.
Figures

References
-
- Saab S., Le L., Saggi S., Sundaram V., Tong M. J., Toward the elimination of hepatitis C in the United States. Hepatology 67, 2449–2459 (2018). - PubMed
-
- World Health Organization , “Global hepatitis report, 2017” (WHO Rep., World Health Organization, Geneva, Switzerland, 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

