Split-TurboID enables contact-dependent proximity labeling in cells
- PMID: 32424107
- PMCID: PMC7275672
- DOI: 10.1073/pnas.1919528117
Split-TurboID enables contact-dependent proximity labeling in cells
Abstract
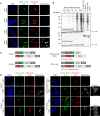
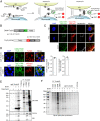
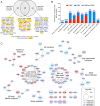
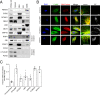
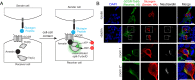
Proximity labeling catalyzed by promiscuous enzymes, such as TurboID, have enabled the proteomic analysis of subcellular regions difficult or impossible to access by conventional fractionation-based approaches. Yet some cellular regions, such as organelle contact sites, remain out of reach for current PL methods. To address this limitation, we split the enzyme TurboID into two inactive fragments that recombine when driven together by a protein-protein interaction or membrane-membrane apposition. At endoplasmic reticulum-mitochondria contact sites, reconstituted TurboID catalyzed spatially restricted biotinylation, enabling the enrichment and identification of >100 endogenous proteins, including many not previously linked to endoplasmic reticulum-mitochondria contacts. We validated eight candidates by biochemical fractionation and overexpression imaging. Overall, split-TurboID is a versatile tool for conditional and spatially specific proximity labeling in cells.
Keywords: ER–mitochondria contacts; proximity labeling; split-TurboID.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
-
- Li P., Li J., Wang L., Di L. J., Proximity labeling of interacting proteins: Application of BioID as a discovery tool. Proteomics 17, 1700002 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

