Large-scale electron microscopy database for human type 1 diabetes
- PMID: 32424134
- PMCID: PMC7235089
- DOI: 10.1038/s41467-020-16287-5
Large-scale electron microscopy database for human type 1 diabetes
Abstract
Autoimmune β-cell destruction leads to type 1 diabetes, but the pathophysiological mechanisms remain unclear. To help address this void, we created an open-access online repository, unprecedented in its size, composed of large-scale electron microscopy images ('nanotomy') of human pancreas tissue obtained from the Network for Pancreatic Organ donors with Diabetes (nPOD; www.nanotomy.org). Nanotomy allows analyses of complete donor islets with up to macromolecular resolution. Anomalies we found in type 1 diabetes included (i) an increase of 'intermediate cells' containing granules resembling those of exocrine zymogen and endocrine hormone secreting cells; and (ii) elevated presence of innate immune cells. These are our first results of mining the database and support recent findings that suggest that type 1 diabetes includes abnormalities in the exocrine pancreas that may induce endocrine cellular stress as a trigger for autoimmunity.
Conflict of interest statement
The authors declare no competing interests.
Figures


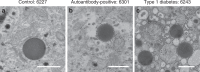
References
-
- Banting FG, Best CH. Pancreatic extracts. 1922. J. Lab Clin. Med. 1990;115:254–272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

