Motor learning promotes remyelination via new and surviving oligodendrocytes
- PMID: 32424285
- PMCID: PMC7329620
- DOI: 10.1038/s41593-020-0637-3
Motor learning promotes remyelination via new and surviving oligodendrocytes
Abstract
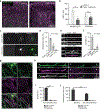
Oligodendrocyte loss in neurological disease leaves axons vulnerable to damage and degeneration, and activity-dependent myelination may represent an endogenous mechanism to improve remyelination following injury. Here we report that, while learning a forelimb reach task transiently suppresses oligodendrogenesis, it subsequently increases oligodendrocyte precursor cell differentiation, oligodendrocyte generation and myelin sheath remodeling in the forelimb motor cortex. Immediately following demyelination, neurons exhibit hyperexcitability, learning is impaired and behavioral intervention provides no benefit to remyelination. However, partial remyelination restores neuronal and behavioral function, allowing learning to enhance oligodendrogenesis, remyelination of denuded axons and the ability of surviving oligodendrocytes to generate new myelin sheaths. Previously considered controversial, we show that sheath generation by mature oligodendrocytes is not only possible but also increases myelin pattern preservation following demyelination, thus presenting a new target for therapeutic interventions. Together, our findings demonstrate that precisely timed motor learning improves recovery from demyelinating injury via enhanced remyelination from new and surviving oligodendrocytes.
Figures








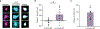

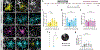





References
-
- Hauser SL, Chan JR & Oksenberg JR Multiple sclerosis: Prospects and promise. Ann. Neurol. 74, 317–327 (2013). - PubMed
-
- Périer O & Grégoire A. Electron microscopic features of multiple sclerosis lesions. Brain J. Neurol. 88, 937–952 (1965). - PubMed
-
- Prineas JW & Connell F. Remyelination in multiple sclerosis. Ann. Neurol. 5, 22–31 (1979). - PubMed
Methods references
-
- Peters A & Sethares C. Oligodendrocytes, their Progenitors and other Neuroglial Cells in the Aging Primate Cerebral Cortex. Cereb. Cortex 14, 995–1007 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

