General anesthetics activate a potent central pain-suppression circuit in the amygdala
- PMID: 32424286
- PMCID: PMC7329612
- DOI: 10.1038/s41593-020-0632-8
General anesthetics activate a potent central pain-suppression circuit in the amygdala
Abstract
General anesthesia (GA) can produce analgesia (loss of pain) independent of inducing loss of consciousness, but the underlying mechanisms remain unclear. We hypothesized that GA suppresses pain in part by activating supraspinal analgesic circuits. We discovered a distinct population of GABAergic neurons activated by GA in the mouse central amygdala (CeAGA neurons). In vivo calcium imaging revealed that different GA drugs activate a shared ensemble of CeAGA neurons. CeAGA neurons also possess basal activity that mostly reflects animals' internal state rather than external stimuli. Optogenetic activation of CeAGA potently suppressed both pain-elicited reflexive and self-recuperating behaviors across sensory modalities and abolished neuropathic pain-induced mechanical (hyper-)sensitivity. Conversely, inhibition of CeAGA activity exacerbated pain, produced strong aversion and canceled the analgesic effect of low-dose ketamine. CeAGA neurons have widespread inhibitory projections to many affective pain-processing centers. Our study points to CeAGA as a potential powerful therapeutic target for alleviating chronic pain.
Conflict of interest statement
Competing Financial Interests Statement
The authors declare no competing financial interests.
Figures
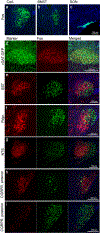
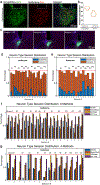



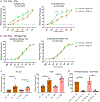
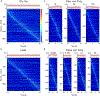
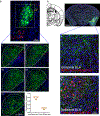
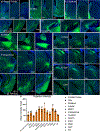







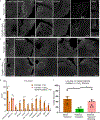
Comment in
-
Anesthesia analgesia in the amygdala.Nat Neurosci. 2020 Jul;23(7):783-785. doi: 10.1038/s41593-020-0645-3. Nat Neurosci. 2020. PMID: 32424288 Free PMC article.
References
-
- Aranake A et al. Increased risk of intraoperative awareness in patients with a history of awareness. Anesthesiology 119, 1275–1283 (2013). - PubMed
-
- Sebel PS et al. The incidence of awareness during anesthesia: a multicenter United States study. Anesth. Analg 99, 833–9, table of contents (2004). - PubMed
-
- Sadove MS, Shulman M, Hatano S & Fevold N Analgesic effects of ketamine administered in subdissociative doses. Anesth. Analg 50, 452–457 (1971). - PubMed
Methods-only References
-
- Venza M et al. The overriding of TRAIL resistance by the histone deacetylase inhibitor MS-275 involves c-myc up-regulation in cutaneous, uveal, and mucosal melanoma. Int. Immunopharmacol 28, 313–321 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

